Phase II Clinical Trial of Neoadjuvant and Adjuvant Pembrolizumab in Resectable Local-Regionally Advanced Head and Neck Squamous Cell Carcinoma
- PMID: 35338369
- PMCID: PMC8976828
- DOI: 10.1158/1078-0432.CCR-21-3351
Phase II Clinical Trial of Neoadjuvant and Adjuvant Pembrolizumab in Resectable Local-Regionally Advanced Head and Neck Squamous Cell Carcinoma
Abstract
Purpose: Patients with resected, local-regionally advanced, head and neck squamous cell carcinoma (HNSCC) have a one-year disease-free survival (DFS) rate of 65%-69% despite adjuvant (chemo)radiotherapy. Neoadjuvant PD-1 immune-checkpoint blockade (ICB) has demonstrated clinical activity, but biomarkers of response and effect on survival remain unclear.
Patients and methods: Eligible patients had resectable squamous cell carcinoma of the oral cavity, larynx, hypopharynx, or oropharynx (p16-negative) and clinical stage T3-T4 and/or two or more nodal metastases or clinical extracapsular nodal extension (ENE). Patients received neoadjuvant pembrolizumab 200 mg 1-3 weeks prior to surgery, were stratified by absence (intermediate-risk) or presence (high-risk) of positive margins and/or ENE, and received adjuvant radiotherapy (60-66 Gy) and concurrent pembrolizumab (every 3 weeks × 6 doses). Patients with high-risk HNSCC also received weekly, concurrent cisplatin (40 mg/m2). Primary outcome was one-year DFS. Secondary endpoints were one-year overall survival (OS) and pathologic response (PR). Safety was evaluated with CTCAE v5.0.
Results: From February 2016 to October 2020, 92 patients enrolled. The median age was 59 years (range, 27-80), 30% were female, 86% had stage T3-T4, and 69% had ≥N2. At a median follow-up of 28 months, one-year DFS was 97% (95% CI, 71%-90%) in the intermediate-risk group and 66% (95% CI, 55%-84%) in the high-risk group. Patients with a PR had significantly improved one-year DFS relative to patients without response (93% vs. 72%, hazard ratio 0.29; 95% CI, 11%-77%). No new safety signals were identified.
Conclusions: Neoadjuvant and adjuvant pembrolizumab increased one-year DFS rate in intermediate-risk, but not high-risk, HNSCC relative to historical control. PR to neoadjuvant ICB is a promising surrogate for DFS.
©2022 American Association for Cancer Research.
Figures
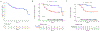


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. - PubMed
-
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. - PubMed
-
- Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843–850. - PubMed

