Efficacy and safety of CD19-specific CAR T cell-based therapy in B-cell acute lymphoblastic leukemia patients with CNSL
- PMID: 35338773
- PMCID: PMC11022988
- DOI: 10.1182/blood.2021013733
Efficacy and safety of CD19-specific CAR T cell-based therapy in B-cell acute lymphoblastic leukemia patients with CNSL
Abstract
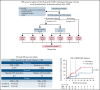
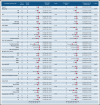
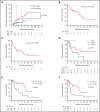
Few studies have described chimeric antigen receptor (CAR) T-cell therapy for patients with B-cell acute lymphoblastic leukemia (B-ALL) with central nervous system leukemia (CNSL) because of concerns regarding poor response and treatment-related neurotoxicity. Our study included 48 patients with relapsed/refractory B-ALL with CNSL to evaluate the efficacy and safety of CD19-specific CAR T cell-based therapy. The infusion resulted in an overall response rate of 87.5% (95% confidence interval [CI], 75.3-94.1) in bone marrow (BM) disease and remission rate of 85.4% (95% CI, 72.8-92.8) in CNSL. With a median follow-up of 11.5 months (range, 1.3-33.3), the median event-free survival was 8.7 months (95% CI, 3.7-18.8), and the median overall survival was 16.0 months (95% CI, 13.5-20.1). The cumulative incidences of relapse in BM and CNS diseases were 31.1% and 11.3%, respectively, at 12 months (P = .040). The treatment was generally well tolerated, with 9 patients (18.8%) experiencing grade ≥3 cytokine release syndrome. Grade 3 to 4 neurotoxic events, which developed in 11 patients (22.9%), were associated with a higher preinfusion disease burden in CNS and were effectively controlled under intensive management. Our results suggest that CD19-specific CAR T cell-based therapy can induce similar high response rates in both BM and CNS diseases. The duration of remission in CNSL was longer than that in BM disease. CD19 CAR T-cell therapy may provide a potential treatment option for previously excluded patients with CNSL, with manageable neurotoxicity. The clinical trials were registered at www.clinicaltrials.gov as #NCT02782351 and www.chictr.org.cn as #ChiCTR-OPN-16008526.
© 2022 by The American Society of Hematology.
Figures


References
-
- Richard-Carpentier G, Kantarjian H, Jabbour E. Recent advances in adult acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2019;14(2):106–118. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

