Platelets accumulate in lung lesions of tuberculosis patients and inhibit T-cell responses and Mycobacterium tuberculosis replication in macrophages
- PMID: 35338775
- PMCID: PMC9325462
- DOI: 10.1002/eji.202149549
Platelets accumulate in lung lesions of tuberculosis patients and inhibit T-cell responses and Mycobacterium tuberculosis replication in macrophages
Abstract
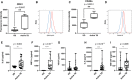
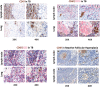
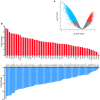
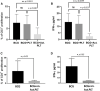
Platelets regulate human inflammatory responses that lead to disease. However, the role of platelets in tuberculosis (TB) pathogenesis is still unclear. Here, we show that patients with active TB have a high number of platelets in peripheral blood and a low number of lymphocytes leading to a high platelets to lymphocytes ratio (PL ratio). Moreover, the serum concentration of different mediators promoting platelet differentiation or associated with platelet activation is increased in active TB. Immunohistochemistry analysis shows that platelets localise around the lung granuloma lesions in close contact with T lymphocytes and macrophages. Transcriptomic analysis of caseous tissue of human pulmonary TB granulomas, followed by Gene Ontology analysis, shows that 53 platelet activation-associated genes are highly expressed compared to the normal lung tissue. In vitro activated platelets (or their supernatants) inhibit BCG-induced T- lymphocyte proliferation and IFN-γ production. Likewise, platelets inhibit the growth of intracellular macrophages of Mycobacterium (M.) tuberculosis. Soluble factors released by activated platelets mediate both immunological and M. tuberculosis replication activities. Furthermore, proteomic and neutralisation studies (by mAbs) identify TGF-β and PF4 as the factors responsible for inhibiting T-cell response and enhancing the mycobactericidal activity of macrophages, respectively. Altogether these results highlight the importance of platelets in TB pathogenesis.
Keywords: Cytokines; Lymphocytes; Macrophages; Mycobacterium tuberculosis; Platelets.
© 2022 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Spatial multiomic profiling reveals the novel polarization of foamy macrophages within necrotic granulomatous lesions developed in lungs of C3HeB/FeJ mice infected with Mycobacterium tuberculosis.Front Cell Infect Microbiol. 2022 Sep 27;12:968543. doi: 10.3389/fcimb.2022.968543. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36237431 Free PMC article.
-
A CD4+CD161+ T-Cell Subset Present in Unexposed Humans, Not Tb Patients, Are Fast Acting Cells That Inhibit the Growth of Intracellular Mycobacteria Involving CD161 Pathway, Perforin, and IFN-γ/Autophagy.Front Immunol. 2021 Feb 26;12:599641. doi: 10.3389/fimmu.2021.599641. eCollection 2021. Front Immunol. 2021. PMID: 33732233 Free PMC article.
-
Platelets Regulate Pulmonary Inflammation and Tissue Destruction in Tuberculosis.Am J Respir Crit Care Med. 2018 Jul 15;198(2):245-255. doi: 10.1164/rccm.201710-2102OC. Am J Respir Crit Care Med. 2018. PMID: 29420060 Free PMC article.
-
Perspectives on host adaptation in response to Mycobacterium tuberculosis: modulation of inflammation.Semin Immunol. 2014 Dec;26(6):533-42. doi: 10.1016/j.smim.2014.10.002. Epub 2014 Oct 25. Semin Immunol. 2014. PMID: 25453228 Review.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
Cited by
-
Platelet-derived microparticles adoptively transfer integrin β3 to promote antitumor effect of tumor-infiltrating T cells.Oncoimmunology. 2024 Jan 16;13(1):2304963. doi: 10.1080/2162402X.2024.2304963. eCollection 2024. Oncoimmunology. 2024. PMID: 38235317 Free PMC article.
-
High levels of PF4, VEGF-A, and classical monocytes correlate with the platelets count and inflammation during active tuberculosis.Front Immunol. 2022 Oct 17;13:1016472. doi: 10.3389/fimmu.2022.1016472. eCollection 2022. Front Immunol. 2022. PMID: 36325331 Free PMC article.
-
Evaluation of platelet indices as markers of tuberculosis among children in India.ERJ Open Res. 2024 Feb 26;10(1):00734-2023. doi: 10.1183/23120541.00734-2023. eCollection 2024 Jan. ERJ Open Res. 2024. PMID: 38410718 Free PMC article.
-
Development of prognostic scoring system for predicting 1-year mortality among pulmonary tuberculosis patients in South India.J Public Health (Oxf). 2023 Jun 14;45(2):e184-e195. doi: 10.1093/pubmed/fdac087. J Public Health (Oxf). 2023. PMID: 36038507 Free PMC article.
-
Full-Blood Inflammatory Ratios Predict Length of Stay but Not Early Death in Romanian Pulmonary Tuberculosis.Medicina (Kaunas). 2025 Jul 9;61(7):1238. doi: 10.3390/medicina61071238. Medicina (Kaunas). 2025. PMID: 40731867 Free PMC article.
References
-
- World Health Organization . Global tuberculosis report 2020. Geneva: World Health Organization; 2020.
-
- Matteelli, A. , Sulis, G. , Capone, S. , D'Ambrosio, L. , Migliori, G. B. and Getahun, H. , Tuberculosis elimination and the challenge of latent tuberculosis. La Presse Médicale 2017. 46( Part 2): e13–e21. - PubMed
-
- Winter, J. R. , Adamu, A. L. , Gupta, R. K. , Stagg, H. R. , Delpech, V. and Abubakar, I. , Tuberculosis infection and disease in people living with HIV in countries with low tuberculosis incidence. Int. J. Tuberc. Lung Dis. 2018. 22: 713–722. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

