Does the timing of saliva collection affect the diagnosis of SARS-CoV-2 infection?
- PMID: 35339382
- PMCID: PMC8948004
- DOI: 10.1016/j.jiac.2022.03.009
Does the timing of saliva collection affect the diagnosis of SARS-CoV-2 infection?
Abstract
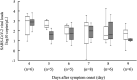
We evaluated the optimal timing of saliva sample collection to diagnose the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We obtained 150 saliva samples at four specific time points from 13 patients with confirmed SARS-CoV-2 infection. The time points were (1) early morning (immediately after waking), (2) immediately after breakfast before tooth brushing, (3) 2 h after breakfast, and (4) before lunch. On the 2nd hospital day, patients collected saliva at the four time points by themselves. We collected samples at two time points, (1) and (3), from the 3rd hospital day to day 9 following symptom onset. In 52 samples collected at the four time points, there was no significant difference. Meanwhile, there was no significant difference in the positive proportion or the viral load between the two time points in both analyses by the day from symptom onset and by all samples. In this study, there was no difference in the positive proportions in saliva collected at various time points within 9 days after symptom onset. The timing of saliva collection was not affected by the diagnosis of SARS-CoV-2 infection.
Keywords: Collection timing; SARS-CoV-2; Saliva.
Copyright © 2022 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare the following conflict of interests which may be considered as potential competing interests: Satoshi Takahashi received speaker honoraria from MSD K.K. and research grants from Shino-Test Corporation, Roche Diagnostic K. K., Fujirebio Inc., and Abbott Japan Co., Ltd. All other authors declare no conflict of interests.
Figures

Similar articles
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of SARS-CoV-2 RNA in Japanese patients with COVID-19.J Infect Chemother. 2021 Jan;27(1):126-129. doi: 10.1016/j.jiac.2020.09.027. Epub 2020 Sep 30. J Infect Chemother. 2021. PMID: 33060046 Free PMC article.
-
Meals and Room Temperature Storage do not Significantly Affect Feasibility of Direct RT-PCR Tests for SARS-CoV-2 Using Saliva.Clin Lab. 2022 Jun 1;68(6). doi: 10.7754/Clin.Lab.2021.210451. Clin Lab. 2022. PMID: 35704722
-
Alternative clinical specimens for the detection of SARS-CoV-2: A rapid review.Rev Med Virol. 2021 Jul;31(4):e2185. doi: 10.1002/rmv.2185. Epub 2020 Oct 22. Rev Med Virol. 2021. PMID: 33091200 Review.
-
Diagnosis of SARS-CoV-2 by RT-PCR Using Different Sample Sources: Review of the Literature.Ear Nose Throat J. 2021 Apr;100(2_suppl):131S-138S. doi: 10.1177/0145561320953231. Epub 2020 Aug 31. Ear Nose Throat J. 2021. PMID: 32865458 Free PMC article. Review.
Cited by
-
Time-of-Day Variation in SARS-CoV-2 RNA Levels during the Second Wave of COVID-19.Viruses. 2022 Aug 5;14(8):1728. doi: 10.3390/v14081728. Viruses. 2022. PMID: 36016350 Free PMC article.
References
-
- Ministry of Health labor and Welfare. COVID-19 tests. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00182.html
-
- Hung D.L., Li X., Chiu K.H., Yip C.C., To K.K., Chan J.F., et al. Early-morning vs spot posterior oropharyngeal saliva for diagnosis of SARS-CoV-2 infection: implication of timing of specimen collection for community-wide screening. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa210. ofaa210. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

