DNA polymerase η promotes nonhomologous end joining upon etoposide exposure dependent on the scaffolding protein Kap1
- PMID: 35339488
- PMCID: PMC9046958
- DOI: 10.1016/j.jbc.2022.101861
DNA polymerase η promotes nonhomologous end joining upon etoposide exposure dependent on the scaffolding protein Kap1
Abstract
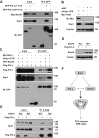
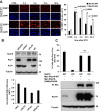
DNA polymerase eta (Pol η) is a eukaryotic member of the Y-family of DNA polymerase involved in translesion DNA synthesis and genome mutagenesis. Recently, several translesion DNA synthesis polymerases have been found to function in repair of DNA double-strand breaks (DSBs). However, the role of Pol η in promoting DSB repair remains to be well defined. Here, we demonstrated that Pol η could be targeted to etoposide (ETO)-induced DSBs and that depletion of Pol η in cells causes increased sensitivity to ETO. Intriguingly, depletion of Pol η also led to a nonhomologous end joining repair defect in a catalytic activity-independent manner. We further identified the scaffold protein Kap1 as a novel interacting partner of Pol η, the depletion of which resulted in impaired formation of Pol η and Rad18 foci after ETO treatment. Additionally, overexpression of Kap1 failed to restore Pol η focus formation in Rad18-deficient cells after ETO treatment. Interestingly, we also found that Kap1 bound to Rad18 in a Pol η-dependent manner, and moreover, depletion of Kap1 led to a significant reduction in Rad18-Pol η association, indicating that Kap1 forms a ternary complex with Rad18 and Pol η to stabilize Rad18-Pol η association. Our findings demonstrate that Kap1 could regulate the role of Pol η in ETO-induced DSB repair via facilitating Rad18 recruitment and stabilizing Rad18-Pol η association.
Keywords: DNA polymerase η; Kap1; Rad18; etoposide; nonhomologous end joining.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Albertella M.R., Green C.M., Lehmann A.R., O'Connor M.J. A role for polymerase eta in the cellular tolerance to cisplatin-induced damage. Cancer Res. 2005;65:9799–9806. - PubMed
-
- Johnson R.E., Prakash S., Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science. 1999;283:1001–1004. - PubMed
-
- Kannouche P.L., Wing J., Lehmann A.R. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: A possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell. 2004;14:491–500. - PubMed
-
- Ma X., Tang T.S., Guo C. Regulation of translesion DNA synthesis in mammalian cells. Environ. Mol. Mutagen. 2020;61:680–692. - PubMed
-
- Arlett C.F., Green M.H., Rogers P.B., Lehmann A.R., Plowman P.N. Minimal ionizing radiation sensitivity in a large cohort of xeroderma pigmentosum fibroblasts. Br. J. Radiol. 2008;81:51–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

