Post-COVID-19 syndrome and humoral response association after 1 year in vaccinated and unvaccinated patients
- PMID: 35339673
- PMCID: PMC8940723
- DOI: 10.1016/j.cmi.2022.03.016
Post-COVID-19 syndrome and humoral response association after 1 year in vaccinated and unvaccinated patients
Abstract
Objectives: This study aimed to describe the impact of vaccination and the role of humoral responses on post-COVID-19 syndrome 1 year after the onset of SARS coronavirus type 2 (CoV-2).
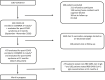
Methods: This prospective study was conducted through interviews to investigate post-COVID-19 syndrome 6 and 12 months after disease onset in all adult in- and outpatients with COVID-19 at Udine Hospital (March-May 2020). Vaccination status and two different serological assays to distinguish between response to vaccination (receptor-binding domain (RBD) SARS-CoV-2 IgG) and/or natural infection (non-RBD-SARS-CoV-2 IgG) were also assessed.
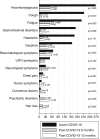
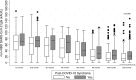
Results: A total of 479 patients (52.6% female; mean age: 53 years) were interviewed 13.5 months (standard deviation: 0.6 months) after acute infection. Post-COVID-19 syndrome was observed in 47.2% of patients (n = 226) after 1 year. There were no significant differences in the worsening of post-COVID-19 symptoms (22.7% vs. 15.8%; p = 0.209) among vaccinated (n = 132) and unvaccinated (n = 347) patients. The presence of non-RBD SARS-CoV-2 IgG induced by natural infection showed a significant association with post-COVID-19 syndrome (OR: 1.35; 95% CI, 1.11-1.64; p = 0.003), and median non-RBD SARS-CoV-2 IgG titres were significantly higher in long haulers than in patients without symptoms (22 kAU/L (interquartile range, 9.7-37.2 kAU/L) vs. 14.1 kAU/L (interquartile range, 5.4-31.3 kAU/L); p = 0.009) after 1 year. In contrast, the presence of RBD SARS-CoV-2 IgG was not associated with the occurrence of post-COVID-19 syndrome (>2500 U/mL vs. 0.9-2500 U/mL; OR: 1.36; 95% CI, 0.62-3.00; p = 0.441), and RBD SARS-CoV-2 IgG titres were similar in long haulers as in patients without symptoms (50% values > 2500 U/mL vs. 55.6% values > 2500 U/mL; p = 0.451).
Discussion: The SARS-CoV-2 vaccination is not associated with the emergence of post-COVID-19 symptoms more than 1 year after acute infection. The persistence of high serological titre response induced by natural infection, but not vaccination, may play a role in long-haul COVID-19.
Keywords: COVID-19 vaccination; Hybrid immunity; Long COVID-19; Natural immunity; Post–COVID-19; SARS-CoV-2 antibodies; SARS-CoV-2 serology; SARS-CoV-2 vaccination; Unvaccinated; Vaccinated.
Copyright © 2022 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Re: Post-COVID-19 syndrome and humoral response association after one year in vaccinated and unvaccinated patients by Peghin et al.Clin Microbiol Infect. 2022 Oct;28(10):1396. doi: 10.1016/j.cmi.2022.04.007. Epub 2022 Apr 21. Clin Microbiol Infect. 2022. PMID: 35462000 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

