UCHL1 protects against ischemic heart injury via activating HIF-1α signal pathway
- PMID: 35339825
- PMCID: PMC8961225
- DOI: 10.1016/j.redox.2022.102295
UCHL1 protects against ischemic heart injury via activating HIF-1α signal pathway
Abstract
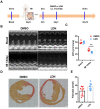
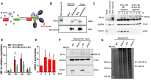
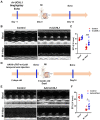
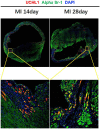
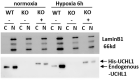
Ubiquitin carboxyl-terminal esterase L1 (UCHL1) has been thought to be a neuron specific protein and shown to play critical roles in Parkinson's Disease and stroke via de-ubiquiting and stabilizing key pathological proteins, such as α-synuclein. In the present study, we found that UCHL1 was significantly increased in both mouse and human cardiomyocytes following myocardial infarction (MI). When LDN-57444, a pharmacological inhibitor of UCHL1, was used to treat mice subjected to MI surgery, we found that administration of LDN-57444 compromised cardiac function when compared with vehicle treated hearts, suggesting a potential protective role of UCHL1 in response to MI. When UCHL1 was knockout by CRISPR/Cas 9 gene editing technique in human induced pluripotent stem cells (hiPSCs), we found that cardiomyocytes derived from UCHL1-/- hiPSCs were more susceptible to hypoxia/re-oxygenation induced injury as compared to wild type cardiomyocytes. To study the potential targets of UCHL1, a BioID based proximity labeling approach followed by mass spectrum analysis was performed. The result suggested that UCHL1 could bind to and stabilize HIF-1α following MI. Indeed, expression of HIF-1α was lower in UCHL1-/- cells as determined by Western blotting and HIF-1α target genes were also suppressed in UCHL1-/- cells as quantified by real time RT-PCR. Recombinant UCHL1 (rUCHL1) protein was purified by E. Coli fermentation and intraperitoneally (I.P.) delivered to mice. We found that administration of rUCHL1 could significantly preserve cardiac function following MI as compared to control group. Finally, adeno associated virus mediated cardiac specific UCHL1 delivery (AAV9-cTNT-m-UCHL1) was performed in neonatal mice. UCHL1 overexpressing hearts were more resistant to MI injury as compare to the hearts infected with control virus. In summary, our data revealed a novel protective role of UCHL1 on MI via stabilizing HIF-1α and promoting HIF-1α signaling.
Keywords: Deubiquitylating enzyme; HIF-1α; Ischemic cardiac injury; UCHL1.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





References
-
- Lawton J.S., Tamis-Holland J.E., Bangalore S., Bates E.R., Beckie T.M., Bischoff J.M., Bittl J.A., Cohen M.G., DiMaio J.M., Don C.W., Fremes S.E., Gaudino M.F., Goldberger Z.D., Grant M.C., Jaswal J.B., Kurlansky P.A., Mehran R., Metkus T.S., Jr., Nnacheta L.C., Rao S.V., Sellke F.W., Sharma G., Yong C.M., Zwischenberger B.A. ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;145:e4–e17. 2022. - PubMed
-
- Writing Committee M., Lawton J.S., Tamis-Holland J.E., Bangalore S., Bates E.R., Beckie T.M., Bischoff J.M., Bittl J.A., Cohen M.G., DiMaio J.M., Don C.W., Fremes S.E., Gaudino M.F., Goldberger Z.D., Grant M.C., Jaswal J.B., Kurlansky P.A., Mehran R., Metkus T.S., Jr., Nnacheta L.C., Rao S.V., Sellke F.W., Sharma G., Yong C.M., Zwischenberger B.A. ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2021;79:197–215. 2022. - PubMed
-
- Buja L.M., Vela D. Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovasc. Pathol. 2008;17:349–374. - PubMed
-
- Lee S.H., Wolf P.L., Escudero R., Deutsch R., Jamieson S.W., Thistlethwaite P.A. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000;342:626–633. - PubMed
-
- Ong S.G., Hausenloy D.J. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol. Ther. 2012;136:69–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

