Novel Small Molecule Fibroblast Growth Factor 23 Inhibitors Increase Serum Phosphate and Improve Skeletal Abnormalities in Hyp Mice
- PMID: 35339985
- PMCID: PMC11033927
- DOI: 10.1124/molpharm.121.000471
Novel Small Molecule Fibroblast Growth Factor 23 Inhibitors Increase Serum Phosphate and Improve Skeletal Abnormalities in Hyp Mice
Abstract
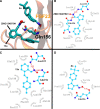
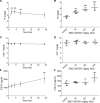
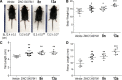
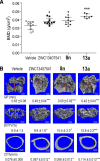
Excess fibroblast growth factor (FGF) 23 causes hereditary hypophosphatemic rickets, such as X-linked hypophosphatemia (XLH) and tumor-induced osteomalacia (TIO). A small molecule that specifically binds to FGF23 to prevent activation of the fibroblast growth factor receptor/α-Klotho complex has potential advantages over the currently approved systemically administered FGF23 blocking antibody. Using structure-based drug design, we previously identified ZINC13407541 (N-[[2-(2-phenylethenyl)cyclopenten-1-yl]methylidene]hydroxylamine) as a small molecule antagonist for FGF23. Additional structure-activity studies developed a series of ZINC13407541 analogs with enhanced drug-like properties. In this study, we tested in a preclinical Hyp mouse homolog of XLH a direct connect analog [(E)-2-(4-(tert-butyl)phenyl)cyclopent-1-ene-1-carbaldehyde oxime] (8n), which exhibited the greatest stability in microsomal assays, and [(E)-2-((E)-4-methylstyryl)benzaldehyde oxime] (13a), which exhibited increased in vitro potency. Using cryo-electron microscopy structure and computational docking, we identified a key binding residue (Q156) of the FGF23 antagonists, ZINC13407541, and its analogs (8n and 13a) in the N-terminal domain of FGF23 protein. Site-directed mutagenesis and bimolecular fluorescence complementation-fluorescence resonance energy transfer assay confirmed the binding site of these three antagonists. We found that pharmacological inhibition of FGF23 with either of these compounds blocked FGF23 signaling and increased serum phosphate and 1,25-dihydroxyvitamin D [1,25(OH)2D] concentrations in Hyp mice. Long-term parenteral treatment with 8n or 13a also enhanced linear bone growth, increased mineralization of bone, and narrowed the growth plate in Hyp mice. The more potent 13a compound had greater therapeutic effects in Hyp mice. Further optimization of these FGF23 inhibitors may lead to versatile drugs to treat excess FGF23-mediated disorders. SIGNIFICANCE STATEMENT: This study used structure-based drug design and medicinal chemistry approaches to identify and optimize small molecules with different stability and potency, which antagonize excessive actions of fibroblast growth factor 23 (FGF23) in hereditary hypophosphatemic rickets. The findings confirmed that these antagonists bind to the N-terminus of FGF23 to inhibit its binding to and activation of the fibroblast growth factor receptors/α-Klotho signaling complex. Administration of these lead compounds improved phosphate homeostasis and abnormal skeletal phenotypes in a preclinical Hyp mouse model.
U.S. Government work not protected by U.S. copyright.
Figures
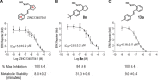



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

