Bacterial meningitis: Aetiology, risk factors, disease trends and severe sequelae during 50 years in Sweden
- PMID: 35340067
- PMCID: PMC9544249
- DOI: 10.1111/joim.13488
Bacterial meningitis: Aetiology, risk factors, disease trends and severe sequelae during 50 years in Sweden
Abstract
Background: Bacterial meningitis (BM) is a rare but severe infection. Few population-based studies have characterised BM episodes and sequelae over long periods.
Methods: This was a population-based observational cohort study with national coverage, using data on aetiological pathogens, sex, premorbid conditions, steroid pretreatment, severe sequelae and birth, death and diagnosis dates collected from 10,339 patients with BM reported to the National Board of Health and Welfare in Sweden between 1964 and 2014.
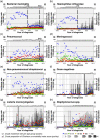
Results: During the 50-year study period, the incidence of BM decreased in young children, but not in the elderly. The most common cause of BM was pneumococci (34%), followed by Haemophilus influenzae (26%), and meningococci (18%), mainly community acquired. Premorbid conditions were found in 20%. After the H. influenzae type b vaccine was introduced in 1993, the BM incidence decreased by 36%. Following pneumococcal conjugated vaccine introduction in 2009, the incidence and 30-day mortality from pneumococcal meningitis decreased by 64% and 100%, respectively, in previously healthy children, and the 30-day mortality decreased by 64% among comorbid adults. The BM incidence in immunosuppressed patients increased by 3% annually post vaccine introduction. The 30-day mortality was 3% in children and 14% in adults, and the rate of severe sequelae was 44%. On average, patients lost 11 years of healthy life due to BM.
Conclusion: The introduction of conjugated vaccines into the childhood vaccination program has reduced the incidence of BM in young children, but not in adults. Post vaccine introduction, patients present with more premorbid conditions and other bacterial causes of BM, emphasising the need for a correct diagnosis when treating these infections.
Keywords: Haemophilus influenzae; Streptococcus pneumoniae; bacterial meningitis; conjugate vaccines; severe sequelae.
© 2022 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


References
-
- Lucas MJ, Brouwer MC, van de Beek D. Neurological sequelae of bacterial meningitis. J Infect. 2016;73(1):18–27. - PubMed
-
- Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328(1):21–8. - PubMed
-
- Laxmi S, Tunkel AR. Healthcare‐associated bacterial meningitis. Curr Infect Dis Rep. 2011;13(4):367–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

