Interest of the Addition of Taxanes to Standard Treatment in First-Line Advanced HER2 Positive Gastroesophageal Adenocarcinoma in Selective Patients
- PMID: 35340264
- PMCID: PMC8948436
- DOI: 10.3389/fonc.2022.763926
Interest of the Addition of Taxanes to Standard Treatment in First-Line Advanced HER2 Positive Gastroesophageal Adenocarcinoma in Selective Patients
Abstract
Background: Studies have reported a beneficial role of the addition of trastuzumab to platin-5-FU based chemotherapy in first-line advanced HER2 positive gastroesophageal adenocarcinoma (GEA). However, the effect of taxanes combined with platin-5FU + trastuzumab (PFT) is understudied.
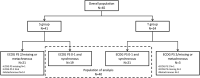
Methods: We performed a retrospective cohort study to evaluate the interest of taxanes among HER2-positive advanced GEA patients treated with PFT. We enrolled HER2-positive advanced GEA patients who underwent treatment between January 2009 to March 2021 in seven hospitals centers in France, treated with PFT alone (S group) or with taxanes + PFT regimen (T group). The primary outcome was progression-free survival (PFS). Also, overall survival (OS), response rate, conversion surgery rate, and safety were evaluated.
Results: Overall, 65 patients received PFT-based therapy, 24 patients in the T group, and 41 patients in the S group. To avoid the selection bias, only those patients presenting an ECOG-PS of 0-1 and synchronous metastasis (21 patients in the T group and 19 patients in the S group) were included for analysis. The median PFS was 9.3 months (95%CI 7.0 to 17.2) in the T group and 5.9 months (95%CI 3.7 to 9.6) in the S group (log-rank p=0.038). Treatment by taxanes was significantly associated with a better PFS in univariate (HR 0.49; 95%CI 0.25 to 0.98, p=0.042) and multivariate Cox regression analysis (HR 0.44; 95%CI 0.21 to 0.94, p=0.033), and IPTW method (HR 0.56; 95% CI 0.34 to 0.91, p=0.019). OS was prolonged (19.0 months (95%CI 7.8 to 45.2) vs 13.0 months (95%CI 5.5 to 14.8), log-rank p=0.033) in favor of the T group. Treatment by taxanes was significantly associated with a better OS in univariate Cox regression analysis (HR 0.49; 95%CI 0.21 to 0.96, p=0.038) and IPTW method (HR 0.49; 95% CI 0.29 to 0.84, p=0.009). The response rate was higher in the T group, with conversion surgery in five patients. No treatment-related death was observed in both groups.
Conclusions: Given the improvement in PFS and OS, the addition of taxanes to standard chemotherapy could be considered as a promising treatment for selected HER2-positive advanced GEA patients, with PS 0-1 and synchronous metastasis (NCT04920747).
Keywords: HER2; gastric carcinoma; metastatic; survival; taxanes; trastuzumab.
Copyright © 2022 Orillard, Henriques, Vernerey, Almotlak, Calcagno, Fein, Fratté, Jary, Klajer, Vienot, Borg and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. . Trastuzumab in Combination With Chemotherapy Versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet (2010) 376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X - DOI - PubMed
-
- Makiyama A, Sukawa Y, Kashiwada T, Kawada J, Hosokawa A, Horie Y, et al. . Randomized, Phase II Study of Trastuzumab Beyond Progression in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT Study). JCO (2020) JCO.19.03077. doi: 10.1200/JCO.19.03077 - DOI - PubMed
-
- Satoh T, Xu R-H, Chung HC, Sun G-P, Doi T, Xu J-M, et al. . Lapatinib Plus Paclitaxel Versus Paclitaxel Alone in the Second-Line Treatment of HER2 -Amplified Advanced Gastric Cancer in Asian Populations: TyTAN—A Randomized, Phase III Study. J Clin Oncol (2014) 32(19):2039–49. doi: 10.1200/JCO.2013.53.6136 - DOI - PubMed
-
- Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. . Trastuzumab Emtansine Versus Taxane Use for Previously Treated HER2-Positive Locally Advanced or Metastatic Gastric or Gastro-Oesophageal Junction Adenocarcinoma (GATSBY): An International Randomised, Open-Label, Adaptive, Phase 2/3 Study. Lancet Oncol (2017) 18(5):640–53. doi: 10.1016/S1470-2045(17)30111-0 - DOI - PubMed
-
- Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. . Pertuzumab Plus Trastuzumab and Chemotherapy for HER2-Positive Metastatic Gastric or Gastro-Oesophageal Junction Cancer (JACOB): Final Analysis of a Double-Blind, Randomised, Placebo-Controlled Phase 3 Study. Lancet Oncol (2018) 19(10):1372–84. doi: 10.1016/S1470-2045(18)30481-9 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

