Self-assembling peptide gels promote angiogenesis and functional recovery after spinal cord injury in rats
- PMID: 35340425
- PMCID: PMC8943448
- DOI: 10.1177/20417314221086491
Self-assembling peptide gels promote angiogenesis and functional recovery after spinal cord injury in rats
Abstract
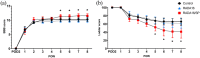
Spinal cord injury (SCI) leads to disruption of the blood-spinal cord barrier, hemorrhage, and tissue edema, which impair blood circulation and induce ischemia. Angiogenesis after SCI is an important step in the repair of damaged tissues, and the extent of angiogenesis strongly correlates with the neural regeneration. Various biomaterials have been developed to promote angiogenesis signaling pathways, and angiogenic self-assembling peptides are useful for producing diverse supramolecular structures with tunable functionality. RADA16 (Ac-RARADADARARADADA-NH2), which forms nanofiber networks under physiological conditions, is a self-assembling peptide that can provide mechanical support for tissue regeneration and reportedly has diverse roles in wound healing. In this study, we applied an injectable form of RADA16 with or without the neuropeptide substance P to the contused spinal cords of rats and examined angiogenesis within the damaged spinal cord and subsequent functional improvement. Histological and immunohistochemical analyses revealed that the inflammatory cell population in the lesion cavity was decreased, the vessel number and density around the damaged spinal cord were increased, and the levels of neurofilaments within the lesion cavity were increased in SCI rats that received RADA16 and RADA16 with substance P (rats in the RADA16/SP group). Moreover, real-time PCR analysis of damaged spinal cord tissues showed that IL-10 expression was increased and that locomotor function (as assessed by the Basso, Beattie, and Bresnahan (BBB) scale and the horizontal ladder test) was significantly improved in the RADA16/SP group compared to the control group. Our findings indicate that RADA16 modified with substance P effectively stimulates angiogenesis within the damaged spinal cord and is a candidate agent for promoting functional recovery post-SCI.
Keywords: Spinal cord injury; angiogenesis; functional recovery; self-assembling peptide; substance P.
© The Author(s) 2022.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






Similar articles
-
Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury.J Neurosurg Spine. 2016 Dec;25(6):745-755. doi: 10.3171/2016.4.SPINE15923. Epub 2016 Jul 1. J Neurosurg Spine. 2016. PMID: 27367940
-
Low-energy extracorporeal shock wave therapy promotes vascular endothelial growth factor expression and improves locomotor recovery after spinal cord injury.J Neurosurg. 2014 Dec;121(6):1514-25. doi: 10.3171/2014.8.JNS132562. Epub 2014 Oct 3. J Neurosurg. 2014. PMID: 25280090
-
Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self-assembling scaffold.PLoS One. 2011;6(5):e19782. doi: 10.1371/journal.pone.0019782. Epub 2011 May 18. PLoS One. 2011. PMID: 21611127 Free PMC article.
-
Design of a RADA16-based self-assembling peptide nanofiber scaffold for biomedical applications.J Biomater Sci Polym Ed. 2019 May-Jun;30(9):713-736. doi: 10.1080/09205063.2019.1605868. Epub 2019 Apr 24. J Biomater Sci Polym Ed. 2019. PMID: 31018781 Review.
-
Modification Strategies for Ionic Complementary Self-Assembling Peptides: Taking RADA16-I as an Example.Polymers (Basel). 2022 Nov 30;14(23):5221. doi: 10.3390/polym14235221. Polymers (Basel). 2022. PMID: 36501615 Free PMC article. Review.
Cited by
-
Lactate promotes microglial scar formation and facilitates locomotor function recovery by enhancing histone H4 lysine 12 lactylation after spinal cord injury.J Neuroinflammation. 2024 Aug 3;21(1):193. doi: 10.1186/s12974-024-03186-5. J Neuroinflammation. 2024. PMID: 39095832 Free PMC article.
-
Mesenchymal stem cells overexpressing XIST induce macrophage M2 polarization and improve neural stem cell homeostatic microenvironment, alleviating spinal cord injury.J Tissue Eng. 2024 Jan 10;15:20417314231219280. doi: 10.1177/20417314231219280. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 38223166 Free PMC article.
-
A Roadmap of Peptide-Based Materials in Neural Regeneration.Adv Healthc Mater. 2025 Jan;14(2):e2402939. doi: 10.1002/adhm.202402939. Epub 2024 Nov 14. Adv Healthc Mater. 2025. PMID: 39540310 Free PMC article. Review.
-
Engineered hydrogels for peripheral nerve repair.Mater Today Bio. 2023 May 19;20:100668. doi: 10.1016/j.mtbio.2023.100668. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37273791 Free PMC article. Review.
-
Exploring the role of two polypeptide nanofiber gels derived from RADA16-I self-assembly in accelerating burn wound healing.Sci Rep. 2025 Jul 8;15(1):24476. doi: 10.1038/s41598-025-08666-z. Sci Rep. 2025. PMID: 40628849 Free PMC article.
References
-
- Kundi S, Bicknell R, Ahmed Z. The role of angiogenic and wound-healing factors after spinal cord injury in mammals. Neurosci Res 2013; 76: 1–9. - PubMed
LinkOut - more resources
Full Text Sources

