Protocol for a Process Evaluation of the Quality Improvement Intervention to Enhance Access to Kidney Transplantation and Living Kidney Donation (EnAKT LKD) Cluster-Randomized Clinical Trial
- PMID: 35340770
- PMCID: PMC8943297
- DOI: 10.1177/20543581221084502
Protocol for a Process Evaluation of the Quality Improvement Intervention to Enhance Access to Kidney Transplantation and Living Kidney Donation (EnAKT LKD) Cluster-Randomized Clinical Trial
Abstract
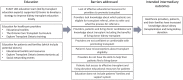
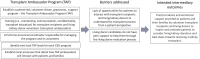
Background: Many patients who would benefit from a kidney transplant never receive one. The Enhance Access to Kidney Transplantation and Living Kidney Donation (EnAKT LKD) pragmatic, cluster-randomized clinical trial is testing whether a multi-component quality improvement intervention, provided in chronic kidney disease (CKD) programs (vs. usual care), can help patients with CKD with no recorded contraindications to kidney transplant complete more steps toward receiving a transplant (primary outcome of the trial). The EnAKT LKD intervention has 4 components: (1) quality Improvement teams and administrative support, (2) improved transplant education for patients and healthcare providers, (3) access to support and (4) program-level performance monitoring.
Objective: To conduct a process evaluation of the EnAKT LKD quality improvement intervention to determine if the components were delivered, received, and enacted as designed (fidelity), and if the intervention addressed intended barriers (mechanisms of change).
Design: A mixed-methods process evaluation informed by new practice implementation and theories of behavior change.
Setting: Chronic kidney disease programs in Ontario, Canada, began receiving the EnAKT LKD intervention on November 1, 2017 and will continue to receive it until December 31, 2021. The process evaluation (interviews and surveys) will occur alongside the trial, between December 2020 to May 2021.
Participants: Healthcare providers (eg, dialysis nurses, nephrologists, members of the multi-care kidney clinic team) at Ontario's 27 CKD programs.
Methods: We will survey and interview healthcare providers at each CKD program, and complete an intervention implementation checklist. Quantitative data from the surveys and the intervention implementation checklist will assess fidelity to the intervention, while quantitative and qualitative data from surveys and interviews will provide insight into the mechanisms of change.
Limitations: The long trial period may result in poor participant recall.
Conclusion: This process evaluation will enhance interpretation of the trial findings, guide improvements in the intervention components, and inform future implementation.
Trial registration: Clinicaltrials.gov; identifier: NCT03329521.
Contexte: Plusieurs patients qui pourraient tirer profit d’une greffe de rein n’en reçoivent jamais une. L’essai clinique pragmatique et randomisé par grappes EnAKT LKD (Enhance Access to Kidney Transplantation and Living Kidney Donation) vise l’amélioration de l’accès à la transplantation rénale et au don de rein vivant. L’essai examine une intervention d’amélioration de la qualité (par rapport aux soins habituels) à composantes multiples réalisée dans le cadre des programs d’insuffisance rénale chronique (IRC) afin de déterminer si elle peut aider les patients atteints d’une néphropathie chronique sans contre-indications documentées à une greffe rénale à franchir davantage d’étapes vers la réception d’une greffe (principal critère d’évaluation de l’essai). L’intervention EAKT LKD comporte quatre composantes : 1) les équipes d’amélioration de la qualité et le soutien administratif; 2) l’amélioration de l’éducation sur la transplantation destinée aux patients et aux fournisseurs de soins; 3) l’accès au soutien; et 4) le suivi du rendement à l’échelle du program.
Objectif: L’évaluation du processus de l’intervention d’amélioration de la qualité EnAKT LKD vise deux objectifs : déterminer si les composants ont été livrés, reçus et mis en œuvre comme prévu (fidélité) et vérifier si l’intervention a permis d’éliminer les obstacles prévus (mécanismes de changement).
Type d’étude: Une évaluation de processus à méthodes mixtes fondée sur les théories concernant la mise en œuvre de nouvelles pratiques et les changements de comportement.
Cadre: Les programs d’IRC ontariens (Canada) ont commencé à recevoir l’intervention EnAKT LKD le 1er novembre 2017 et ont continué de la recevoir jusqu’au 31 décembre 2021. L’évaluation du processus (sondages et entretiens) s’est effectuée parallèlement à l’essai, de décembre 2020 à mai 2021.
Participants: Les fournisseurs de soins (infirmières en dialyze, néphrologues, membres du personnel des cliniques multidisciplinaires en santé rénale) des 27 programs d’IRC ontariens.
Méthodologie: Nous allons sonder et interroger les fournisseurs de soins de chaque program d’IRC et nous complèterons une liste vérifiant la mise en œuvre de l’intervention. Les données quantitatives tirées des sondages et listes de vérification permettront d’évaluer la fidélité à l’intervention, alors que les données quantitatives et qualitatives extraites des sondages et des entretiens fourniront un aperçu des mécanismes de changement.
Limites: La longue période de l’essai pourrait rendre difficile le rappel des participants.
Conclusion: Cette évaluation du processus permettra d’améliorer l’interprétation des résultats de l’essai et de guider l’amélioration des composantes de l’intervention, en plus d’éclairer de futures mises en œuvre.
Enregistrement de l’essai: ClinicalTrials.gov; identifiant : NCT03329521.
Keywords: chronic kidney disease; fidelity; kidney transplantation; living kidney donation; mechanisms of change; normalization process theory; process evaluation; protocol; theoretical domains framework.
© The Author(s) 2022.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




Similar articles
-
Enhance Access to Kidney Transplantation and Living Kidney Donation (EnAKT LKD): Statistical Analysis Plan of a Registry-Based, Cluster-Randomized Clinical Trial.Can J Kidney Health Dis. 2022 Nov 22;9:20543581221131201. doi: 10.1177/20543581221131201. eCollection 2022. Can J Kidney Health Dis. 2022. PMID: 36438439 Free PMC article.
-
Effect of a Novel Multicomponent Intervention to Improve Patient Access to Kidney Transplant and Living Kidney Donation: The EnAKT LKD Cluster Randomized Clinical Trial.JAMA Intern Med. 2023 Dec 1;183(12):1366-1375. doi: 10.1001/jamainternmed.2023.5802. JAMA Intern Med. 2023. PMID: 37922156 Free PMC article.
-
Process Evaluation Alongside a Cluster-Randomized Trial of a Multicomponent Intervention Designed to Improve Patient Access to Kidney Transplantation.Can J Kidney Health Dis. 2025 Mar 17;12:20543581251323959. doi: 10.1177/20543581251323959. eCollection 2025. Can J Kidney Health Dis. 2025. PMID: 40104388 Free PMC article.
-
A Quality Improvement Intervention to Enhance Access to Kidney Transplantation and Living Kidney Donation (EnAKT LKD) in Patients With Chronic Kidney Disease: Clinical Research Protocol of a Cluster-Randomized Clinical Trial.Can J Kidney Health Dis. 2021 Apr 15;8:2054358121997266. doi: 10.1177/2054358121997266. eCollection 2021. Can J Kidney Health Dis. 2021. PMID: 33948191 Free PMC article.
-
Sex and Gender Disparities in Living Kidney Donation: A Scoping Review.Transplant Direct. 2023 Aug 24;9(9):e1530. doi: 10.1097/TXD.0000000000001530. eCollection 2023 Sep. Transplant Direct. 2023. PMID: 37636486 Free PMC article.
Cited by
-
Enhance Access to Kidney Transplantation and Living Kidney Donation (EnAKT LKD): Statistical Analysis Plan of a Registry-Based, Cluster-Randomized Clinical Trial.Can J Kidney Health Dis. 2022 Nov 22;9:20543581221131201. doi: 10.1177/20543581221131201. eCollection 2022. Can J Kidney Health Dis. 2022. PMID: 36438439 Free PMC article.
-
Effect of a Novel Multicomponent Intervention to Improve Patient Access to Kidney Transplant and Living Kidney Donation: The EnAKT LKD Cluster Randomized Clinical Trial.JAMA Intern Med. 2023 Dec 1;183(12):1366-1375. doi: 10.1001/jamainternmed.2023.5802. JAMA Intern Med. 2023. PMID: 37922156 Free PMC article.
-
Process Evaluation Alongside a Cluster-Randomized Trial of a Multicomponent Intervention Designed to Improve Patient Access to Kidney Transplantation.Can J Kidney Health Dis. 2025 Mar 17;12:20543581251323959. doi: 10.1177/20543581251323959. eCollection 2025. Can J Kidney Health Dis. 2025. PMID: 40104388 Free PMC article.
References
-
- Tonelli M, Wiebe N, Knoll G, et al.. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093-2109. - PubMed
-
- Rana A, Gruessner A, Agopian VG, et al.. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252-259. - PubMed
-
- Ortiz F, Aronen P, Koskinen PK, et al.. Health-related quality of life after kidney transplantation: who benefits the most. Transpl Int. 2014;27(11):1143-1151. - PubMed
-
- Canadian Institute for Health Information. Annual Statistics on Organ Replacement in Canada: Dialysis, Transplantation and Donation, 2008 to 2017. Ottawa, ON: Canadian Institute for Health Information; 2018.
-
- Horvat LD, Shariff SZ, Garg AX, Donor Nephrectomy Outcomes Research (DONOR) Network. Global trends in the rates of living kidney donation. Kidney Int. 2009;75(10):1088-1098. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

