Cooperativity as quantification and optimization paradigm for nuclear receptor modulators
- PMID: 35340861
- PMCID: PMC8890100
- DOI: 10.1039/d1sc06426f
Cooperativity as quantification and optimization paradigm for nuclear receptor modulators
Abstract
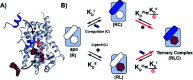
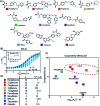
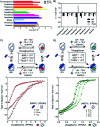
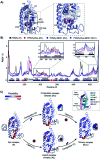
Nuclear Receptors (NRs) are highly relevant drug targets, for which small molecule modulation goes beyond a simple ligand/receptor interaction. NR-ligands modulate Protein-Protein Interactions (PPIs) with coregulator proteins. Here we bring forward a cooperativity mechanism for small molecule modulation of NR PPIs, using the Peroxisome Proliferator Activated Receptor γ (PPARγ), which describes NR-ligands as allosteric molecular glues. The cooperativity framework uses a thermodynamic model based on three-body binding events, to dissect and quantify reciprocal effects of NR-coregulator binding (K I D) and NR-ligand binding (K II D), jointly recapitulated in the cooperativity factor (α) for each specific ternary ligand·NR·coregulator complex formation. These fundamental thermodynamic parameters allow for a conceptually new way of thinking about structure-activity-relationships for NR-ligands and can steer NR modulator discovery and optimization via a completely novel approach.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

