Ganoderic acid A ameliorates non-alcoholic streatohepatitis (NASH) induced by high-fat high-cholesterol diet in mice
- PMID: 35340879
- PMCID: PMC8931630
- DOI: 10.3892/etm.2022.11237
Ganoderic acid A ameliorates non-alcoholic streatohepatitis (NASH) induced by high-fat high-cholesterol diet in mice
Abstract
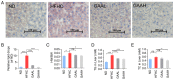
Non-alcoholic steatohepatitis (NASH) is becoming a huge global health problem. Previous studies have revealed that ganoderic acids have hepatoprotective and hypocholesterolemic effects. In the present study, to evaluate the anti-NASH activity of ganoderic acid A (GAA), male 6-week-old C57BL/6J mice were divided into the following four groups, which were administered different diets: Normal diet (ND group), high-fat high-cholesterol diet (HFHC group), HFHC diet supplemented with 25 mg/kg/day (GAAL group) or 50 mg/kg/day of GAA (GAAH group). After 12 weeks of GAA treatment, histopathological results revealed that compared with that of the HFHC group, GAA significantly inhibited fat accumulation, steatosis, inflammation and fibrosis in the liver. GAA effectively reduced serum aspartate transaminase and alanine transaminase levels compared with the HFHC model. Furthermore, the endoplasmic reticulum (ER) stress-responsive proteins, including glucose-regulated protein 78, phosphorylated (p)-eukaryotic initiation factor-2α and p-JNK, were significantly suppressed by GAA, while ERp57, p-MAPK and p-AKT were significantly increased after GAA treatment. Taken together, it was concluded that GAA could resist HFHC diet-induced NASH. In terms of its underlying mechanism, GAA could improve liver inflammation and fibrosis by inhibiting hepatic oxidative stress and the ER stress response induced by HFHC.
Keywords: endoplasmic reticulum stress; ganoderic acid A; high-fat high-cholesterol diet; inflammation; non-alcoholic streatohepatitis.
Copyright: © Zhu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
