Glycolytic metabolism and activation of Na+ pumping contribute to extracellular acidification in the central clock of the suprachiasmatic nucleus: Differential glucose sensitivity and utilization between oxidative and non-oxidative glycolytic pathways
- PMID: 35341719
- PMCID: PMC9133309
- DOI: 10.1016/j.bj.2021.02.004
Glycolytic metabolism and activation of Na+ pumping contribute to extracellular acidification in the central clock of the suprachiasmatic nucleus: Differential glucose sensitivity and utilization between oxidative and non-oxidative glycolytic pathways
Abstract
Background: The central clock of the suprachiasmatic nucleus (SCN) controls the metabolism of glucose and is sensitive to glucose shortage. However, it is only beginning to be understood how metabolic signals such as glucose availability regulate the SCN physiology. We previously showed that the ATP-sensitive K+ channel plays a glucose-sensing role in regulating SCN neuronal firing at times of glucose shortage. Nevertheless, it is unknown whether the energy-demanding Na+/K+-ATPase (NKA) is also sensitive to glucose availability. Furthermore, we recently showed that the metabolically active SCN constantly extrudes H+ to acidify extracellular pH (pHe). This study investigated whether the standing acidification is associated with Na+ pumping activity, energy metabolism, and glucose utilization, and whether glycolysis- and mitochondria-fueled NKAs may be differentially sensitive to glucose shortage.
Methods: Double-barreled pH-selective microelectrodes were used to determine the pHe in the SCN in hypothalamic slices.
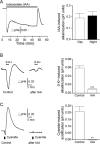
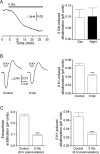
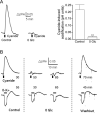
Results: NKA inhibition with K+-free (0-K+) solution rapidly and reversibly alkalinized the pHe, an effect abolished by ouabain. Mitochondrial inhibition with cyanide acidified the pHe but did not inhibit 0-K+-induced alkalinization. Glycolytic inhibition with iodoacetate alkalinized the pHe, completely blocked cyanide-induced acidification, and nearly completely blocked 0-K+-induced alkalinization. The results indicate that glycolytic metabolism and activation of Na+ pumping contribute to the standing acidification. Glucoprivation also alkalinized the pHe, nearly completely eliminated cyanide-induced acidification, but only partially reduced 0-K+-induced alkalinization. In contrast, hypoglycemia preferentially and partially blocked cyanide-induced acidification. The result indicates sensitivity to glucose shortage for the mitochondria-associated oxidative glycolytic pathway.
Conclusion: Glycolytic metabolism and activation of glycolysis-fueled NKA Na+ pumping activity contribute to the standing acidification in the SCN. Furthermore, the oxidative and non-oxidative glycolytic pathways differ in their glucose sensitivity and utilization, with the oxidative glycolytic pathway susceptible to glucose shortage, and the non-oxidative glycolytic pathway able to maintain Na+ pumping even in glucoprivation.
Keywords: Glycolysis; Metabolism; Na(+)/K(+)-ATPase; Suprachiasmatic nucleus; pH.
Copyright © 2021 Chang Gung University. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures



Similar articles
-
Intracellular Na(+) and metabolic modulation of Na/K pump and excitability in the rat suprachiasmatic nucleus neurons.J Neurophysiol. 2012 Oct;108(7):2024-32. doi: 10.1152/jn.00361.2012. Epub 2012 Jul 5. J Neurophysiol. 2012. PMID: 22773774
-
The Na+/H+-Exchanger NHE1 Regulates Extra- and Intracellular pH and Nimodipine-sensitive [Ca2+]i in the Suprachiasmatic Nucleus.Sci Rep. 2019 Apr 23;9(1):6430. doi: 10.1038/s41598-019-42872-w. Sci Rep. 2019. PMID: 31015514 Free PMC article.
-
KATP Channels Mediate Differential Metabolic Responses to Glucose Shortage of the Dorsomedial and Ventrolateral Oscillators in the Central Clock.Sci Rep. 2017 Apr 4;7(1):640. doi: 10.1038/s41598-017-00699-3. Sci Rep. 2017. PMID: 28377630 Free PMC article.
-
Differential regulation of nimodipine-sensitive and -insensitive Ca2+ influx by the Na+/Ca2+ exchanger and mitochondria in the rat suprachiasmatic nucleus neurons.J Biomed Sci. 2018 May 22;25(1):44. doi: 10.1186/s12929-018-0447-z. J Biomed Sci. 2018. PMID: 29788971 Free PMC article.
-
Energy metabolism and transduction in smooth muscle.Experientia. 1985 Aug 15;41(8):970-7. doi: 10.1007/BF01952116. Experientia. 1985. PMID: 2990994 Review.
Cited by
-
Orthostasis Is Impaired Due to Fatiguing Intensive Acute Concentric Exercise Succeeded by Isometric Weight-Loaded Wall-Sit in Delayed-Onset Muscle Soreness: A Pilot Study.Sports (Basel). 2023 Oct 27;11(11):209. doi: 10.3390/sports11110209. Sports (Basel). 2023. PMID: 37999426 Free PMC article.
-
About gladiators and a sacred disease.Biomed J. 2022 Feb;45(1):1-8. doi: 10.1016/j.bj.2022.03.006. Epub 2022 Mar 23. Biomed J. 2022. PMID: 35339730 Free PMC article.
-
Efficient ocular delivery of siRNA via pH-sensitive vehicles for corneal neovascularization inhibition.Int J Pharm X. 2023 Apr 29;5:100183. doi: 10.1016/j.ijpx.2023.100183. eCollection 2023 Dec. Int J Pharm X. 2023. PMID: 37234133 Free PMC article.
References
-
- Dibner C., Schibler U., Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. - PubMed
-
- Schwartz W.J., Gainer H. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977;197:1089–1091. - PubMed
-
- López L., Lorente L., Arias J., González-Pardo H., Cimadevilla J., Arias J.L. Changes of cytochrome oxidase activity in rat suprachiasmatic nucleus. Brain Res. 1997;769:367–371. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

