Progressive skeletal defects caused by Kindlin3 deficiency, a model of autosomal recessive osteopetrosis in humans
- PMID: 35342016
- PMCID: PMC9133165
- DOI: 10.1016/j.bone.2022.116397
Progressive skeletal defects caused by Kindlin3 deficiency, a model of autosomal recessive osteopetrosis in humans
Abstract
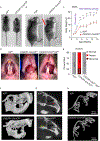
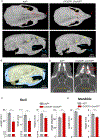
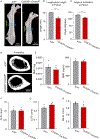
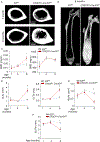
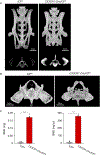
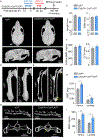
The cellular and molecular mechanisms of bone development and homeostasis are clinically important, but not fully understood. Mutations in integrins and Kindlin3 in humans known as Leukocyte adhesion deficiencies (LAD) cause a wide spectrum of complications, including osteopetrosis. Yet, the rarity, frequent misdiagnosis, and lethality of LAD preclude mechanistic analysis of skeletal abnormalities in these patients. Here, using inducible and constitutive tissue-specific Kindlin3 knockout (K3KO) mice, we show that the constitutive lack of embryonic-Kindlin3 in myeloid lineage cells causes growth retardation, edentulism, and skull deformity indicative of hydrocephaly. Micro-CT analysis revealed craniosynostosis, choanal stenosis, and micrognathia along with other skeletal abnormalities characteristic of osteopetrosis. A marked progression of osteosclerosis occurs in mature to middle-aged adults, resulting in the narrowing of cranial nerve foramina and bone marrow cavities of long bones. However, postnatal-Kindlin3 is less critical for bone remodeling and architecture. Thus, myeloid Kindlin3 is essential for skeletal development and its deficiency leads to autosomal recessive osteopetrosis (ARO). The study will aid in the diagnosis, management, and treatment choices for patients with LAD-III and ARO.
Keywords: Bone analysis; Computerized tomography (CT); Integrins; LAD-III; Osteopetrosis; Teeth.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
1.9 Conflict of interest
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

