Management of immune thrombocytopenia: 2022 update of Korean experts recommendations
- PMID: 35342042
- PMCID: PMC8958378
- DOI: 10.5045/br.2022.2022043
Management of immune thrombocytopenia: 2022 update of Korean experts recommendations
Abstract
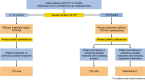
Despite the availability of therapies to treat patients with immune thrombocytopenia (ITP), there is currently little data from randomized trials to assist clinicians in managing patients. The evidence-based guidelines of the Korean Society of Hematology Aplastic Anemia Working Party (KSHAAWP) are intended to support patients and physicians in the management of ITP. Experts from the KSHAAWP discussed and described this guideline according to the current treatment situation for ITP in Korea and finalized the guidelines. The expert panel recommended the management of ITP in adult and pediatric patients with newly diagnosed, persistent, and chronic disease refractory to first-line therapy with minor bleeding. Management approaches include observation and administration of corticosteroids, intravenous immunoglobulin, anti-D immunoglobulin, and thrombopoietin receptor agonists. Currently, evidence supporting strong recommendations for various management approaches is lacking. Therefore, a large focus was placed on shared decision-making, especially regarding second-line treatment.
Keywords: Immune thrombocytopenia; Management; Recommendation.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources