Alzheimer's Disease: Key Insights from Two Decades of Clinical Trial Failures
- PMID: 35342092
- PMCID: PMC9198803
- DOI: 10.3233/JAD-215699
Alzheimer's Disease: Key Insights from Two Decades of Clinical Trial Failures
Abstract
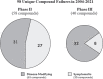
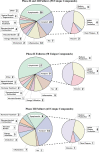
Given the acknowledged lack of success in Alzheimer's disease (AD) drug development over the past two decades, the objective of this review was to derive key insights from the myriad failures to inform future drug development. A systematic and exhaustive review was performed on all failed AD compounds for dementia (interventional phase II and III clinical trials from ClinicalTrials.gov) from 2004 to the present. Starting with the initial ∼2,700 AD clinical trials, ∼550 trials met our initial criteria, from which 98 unique phase II and III compounds with various mechanisms of action met our criteria of a failed compound. The two recent reported phase III successes of aducanumab and oligomannate are very encouraging; however, we are awaiting real-world validation of their effectiveness. These two successes against the 98 failures gives a 2.0% phase II and III success rate since 2003, when the previous novel compound was approved. Potential contributing methodological factors for the clinical trial failures were categorized into 1) insufficient evidence to initiate the pivotal trials, and 2) pivotal trial design shortcomings. Our evaluation found that rational drug development principles were not always followed for AD therapeutics development, and the question remains whether some of the failed compounds may have shown efficacy if the principles were better adhered to. Several recommendations are made for future AD therapeutic development. The whole database of the 98 failed compounds is presented in the Supplementary Material.
Keywords: Alzheimer’s disease; amyloid; biomarkers; clinical trial; dementia; drug development.
Conflict of interest statement
Authors’ disclosures available online (
Figures
References
-
- Alzheimer’s Association (2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15, 321–387.
-
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Prim 1, 15056. - PubMed
-
- Veitch DP, Weiner MW, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR Jr, Jagust W, Morris JC, Petersen RC, Saykin AJ, Shaw LM, Toga AW, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative (2019) Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 15, 106–152. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous