Forsythoside A Mitigates Alzheimer's-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation
- PMID: 35342364
- PMCID: PMC8935224
- DOI: 10.7150/ijbs.69714
Forsythoside A Mitigates Alzheimer's-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation
Abstract
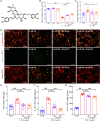
Ferroptosis and neuroinflammation play crucial roles in Alzheimer's disease (AD) pathophysiology. Forsythoside A (FA), the main constituent of Forsythia suspensa (Thunb.) Vahl., possesses anti-inflammatory, antibacterial, antioxidant, and neuroprotective properties. The present study aimed to investigate the potential role of FA in AD neuropathology using male APP/PS1 double transgenic AD mice, Aβ1-42-exposed N2a cells, erastin-stimulated HT22 cells, and LPS-induced BV2 cells. FA treatment significantly improved mitochondrial function and inhibited lipid peroxidation in Aβ1-42-exposed N2a cells. In LPS-stimulated BV2 cells, FA treatment decreased the formation of the pro-inflammatory factors IL-6, IL-1β, and NO. In male APP/PS1 mice, FA treatment ameliorated memory and cognitive impairments and suppressed Aβ deposition and p-tau levels in the brain. Analyses using proteomics, immunohistochemistry, ELISA, and western blot revealed that FA treatment significantly augmented dopaminergic signaling, inhibited iron deposition and lipid peroxidation, prevented the activation of IKK/IκB/NF-κB signaling, reduced the secretion of pro-inflammatory factors, and promoted the production of anti-inflammatory factors in the brain. FA treatment exerted anti-ferroptosis and anti-neuroinflammatory effects in erastin-stimulated HT22 cells, and the Nrf2/GPX4 axis played a key role in these effects. Collectively, these results demonstrate the protective effects of FA and highlight its therapeutic potential as a drug component for AD treatment.
Keywords: Alzheimer's disease; Nrf2/GPX4 axis; ferroptosis; forsythoside A; neuroinflammation; neuroprotection.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

