Resource limitation has a limited impact on the outcome of virus-fungus co-infection in an insect host
- PMID: 35342581
- PMCID: PMC8928876
- DOI: 10.1002/ece3.8707
Resource limitation has a limited impact on the outcome of virus-fungus co-infection in an insect host
Abstract
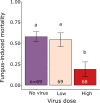
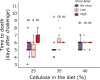
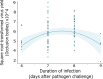
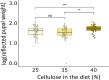
Infection by pathogens is strongly affected by the diet or condition of the prospective host. Studies that examine the impact of diet have mainly focused on single pathogens; however, co-infections within a single host are thought to be common. Different pathogen groups might respond differently to resource availability and diverse infections could increase the costs of host defense, meaning the outcome of mixed infections under varying dietary regimes is likely to be hard to predict. We used the generalist cabbage looper, Trichoplusia ni and two of its pathogens, the DNA virus T. ni nucleopolyhedrovirus (TniSNPV) and the entomopathogenic fungus, Beauveria bassiana to examine how nutrient reduction affected the outcome of mixed pathogen infection. We challenged insects with a low or high effective dose of virus, alone or combined with a single dose of fungus. We manipulated food availability after pathogen challenge by diluting artificial diet with cellulose, a non-nutritious bulking agent, and examined its impact on host and pathogen fitness. Reducing diet quantity did not alter overall or pathogen-specific mortality. In all cases, TniSNPV-induced mortality was negatively affected by fungus challenge. Similarly, B. bassiana-induced mortality was negatively affected by TniSNPV challenge, but only at the higher virus dose. Dietary dilution mainly affected B. bassiana speed of kill when mixed with a high dose of TniSNPV, with an increase in the duration of fungal infection when cellulose was low (high quantity). One pathogen dominated the production of transmission stages in the cadavers and co-infection did not affect the yield of either pathogen. There was no evidence that co-infections were more costly to the survivors of pathogen challenge. In conclusion, dietary dilution did not determine the outcome of mixed pathogen infection, but it had more subtle effects, that differed between the two pathogens and could potentially alter pathogen recycling and host-pathogen dynamics.
Keywords: Beauveria bassiana; baculovirus; host nutrition; mixed infection; pathogen replication; trade‐offs.
© 2022 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
We declare no competing interests.
Figures

References
-
- Cory, J. S. (2010). The ecology of baculoviruses. In Asgari S. & Johnson K. (Eds.), Insect virology (pp. 411–427). Caister Academic Press.
LinkOut - more resources
Full Text Sources

