Transcatheter Closure of Perimembranous Ventricular Septal Defect Using the Lifetech Konar-Multi Functional Occluder: Early to Midterm Results of the Indonesian Multicenter Study
- PMID: 35342698
- PMCID: PMC8877696
- DOI: 10.5334/gh.1106
Transcatheter Closure of Perimembranous Ventricular Septal Defect Using the Lifetech Konar-Multi Functional Occluder: Early to Midterm Results of the Indonesian Multicenter Study
Abstract
Background: The alternative device to close perimembranous ventricular septal defect (pmVSD) has been searched for better result, less complications and applicable for infants. However, the ideal device is still unavailable. We aimed to evaluate the effectiveness and outcome of transcatheter pmVSD closure using the KONAR-multi functional occluder (MFO).
Methods: Clinical, procedural, follow-up data of pmVSD patients with symptom of heart failure or evidence of significant left to right shunt, growth failure, recurrent respiratory tract infection, and history of endocarditis who underwent transcatheter closure using the MFO were prospectively evaluated.
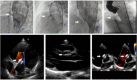
Results: Between January 2016 and December 2017, there were complete records of 132 pmVSD children closed using MFO from eleven centers in Indonesia. The median of age was 4.5 (0.3-17.4) years; weight 14.8 (3.5-57) kg, defect size at the smallest part 3.4 (1.0-8.1) mm, flow ratio 1.6 (1.3-4.9), mean pulmonary artery pressure 18 (7-79) mmHg, fluoroscopy time 18 (3.8-91) and procedural time 75 (26-290) minutes. A retrograde approach was done in 41 (31%) patients. Procedures succeeded in first attempt in 126 (95.4%), failed in three and migration in three patients. Six of eight infants with congestive heart failure were closed successfully. Of 126 patients with successful VSD closure, 12 months follow-up were completed in all patients. The rate of complete occlusion at 1 month, 3 months, 6 months and 12 months after intervention were 95.2%, 97.6%, 99.2%, and 99.2%, respectively. New-onset aortic regurgitation and moderate tricuspid regurgitation developed only in five and three patients. Neither complete atrioventricular block, nor other complications occurred.
Conclusion: Transcatheter closure of pmVSD using the MFO is safe, effective, and feasible in infants and children.
Keywords: KONAR-multi functional occluder; Perimembranous ventricular septal defect; transcatheter closure.
Copyright: © 2022 The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

