Updating estimates of the number of UK stroke patients eligible for endovascular thrombectomy: incorporating recent evidence to facilitate service planning
- PMID: 35342815
- PMCID: PMC8948519
- DOI: 10.1177/23969873211059471
Updating estimates of the number of UK stroke patients eligible for endovascular thrombectomy: incorporating recent evidence to facilitate service planning
Erratum in
-
ERRATUM to Updating estimates of the number of UK stroke patients eligible for endovascular thrombectomy: incorporating recent evidence to facilitate service planning.Eur Stroke J. 2023 Mar;8(1):401. doi: 10.1177/23969873221133911. Epub 2022 Oct 26. Eur Stroke J. 2023. PMID: 37021172 Free PMC article.
Abstract
Introduction: Endovascular thrombectomy (EVT) is a highly effective treatment for acute ischaemic stroke due to large arterial occlusion (LAO). To support decisions about service provision, we previously estimated the annual UK population eligible for EVT as ∼10% of stroke admissions. Since then, several trials have produced evidence that could alter these figures. We update our estimates considering information from studies and trials reporting 2018-2021 on incidence, presentation time and stroke severity and consider the possible impact of predicted demographic changes in the next 10-20 years.
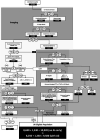
Patients and methods: We produce an updated decision tree describing the EVT eligible population for UK stroke admissions. One-way sensitivity analyses (using upper and lower confidence intervals for estimates at each branch of our decision tree) were used to identify where further research evidence is necessary to increase certainty around estimates for numbers of EVT eligible patients.
Results: The updated estimate for the number of UK stroke patients eligible for EVT annually was between 10,020 (no advanced imaging in early presenting patients) and 9,580 (advanced imaging in all early presenting patients), which compared with our estimates in 2017 is a minimal reduction. One-way sensitivity analyses established that enhanced evidence about eligibility for milder strokes, ASPECTS scores and pre-stroke disability are offset by evidence regarding a lower incidence of LAO. Importantly, predicted increases in life expectancy by 2040 may increase thrombectomy need by 40%.
Discussion: Information from additional randomised trials published during 2018-2020 with updated estimates of LAO prevalence had a minimal impact on overall estimates of stroke patients eligible for EVT in the UK. Ongoing research into the benefits of EVT for patients with mild stroke or European Stroke Journal For Peer Review lower ASPECTS scores has the potential to increase the estimates of the eligible population; future need for EVT will increase with the ageing population.
Conclusion: Our updated analyses show overall numbers eligible little changed, but evidence from ongoing trials and demographic changes have the potential to increase the need for EVT significantly.
Keywords: Thrombectomy; advanced imaging; eligibility; ischaemic stroke; service planning.
© European Stroke Organisation 2021.
Conflict of interest statement
Declaration of conflicting interests: PW was co-PI for two randomised trials (PISTE and STABILISE) investigating different aspects of thrombectomy in acute stroke. Start-up phase of PISTE was mainly funded by Stroke Association but was also part funded by unrestricted institutional educational grants from Covidien and Codman who both manufacture devices used for stroke thrombectomy. STABILISE was part funded by Microvention. PW has also undertaken educational consultancy work within last 3 years for Microvention who manufacture devices used for stroke thrombectomy. He holds institutional unrestricted educational grants for a reperfusion masterclass from Stryker, Penumbra and Medtronic. GAF was co-PI for STABILISE and was chair of the PISTE Trial Steering Committee, both trials involving thrombectomy devices. GAF has received personal remuneration for educational and advisory work from Medtronic and Stryker. MAJ has received personal fees and non-financial support from Boehringer Ingelheim, Bayer, Bristol-Myers-Squibb and Daiichi-Sankyo outside the submitted work.
Figures

References
-
- Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl J Med 2015; 372: 11–20. - PubMed
-
- Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl J Med 2015; 372: 1009–1018. - PubMed
-
- Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl J Med 2015; 372: 1019–1030. - PubMed
-
- Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New Engl J Med 2015; 372: 2285–2295. - PubMed
-
- Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. New Engl J Med 2015; 372: 2296–2306. - PubMed
LinkOut - more resources
Full Text Sources

