Evaluation of immunologic parameters in canine glioma patients treated with an oncolytic herpes virus
- PMID: 35342877
- PMCID: PMC8955901
- DOI: 10.20517/jtgg.2021.31
Evaluation of immunologic parameters in canine glioma patients treated with an oncolytic herpes virus
Abstract
Aim: To molecularly characterize the tumor microenvironment and evaluate immunologic parameters in canine glioma patients before and after treatment with oncolytic human IL-12-expressing herpes simplex virus (M032) and in treatment naïve canine gliomas.
Methods: We assessed pet dogs with sporadically occurring gliomas enrolled in Stage 1 of a veterinary clinical trial that was designed to establish the safety of intratumoral oncoviral therapy with M032, a genetically modified oncolytic herpes simplex virus. Specimens from dogs in the trial and dogs not enrolled in the trial were evaluated with immunohistochemistry, NanoString, Luminex cytokine profiling, and multi-parameter flow cytometry.
Results: Treatment-naive canine glioma microenvironment had enrichment of Iba1 positive macrophages and minimal numbers of T and B cells, consistent with previous studies identifying these tumors as immunologically "cold". NanoString mRNA profiling revealed enrichment for tumor intrinsic pathways consistent with suppression of tumor-specific immunity and support of tumor progression. Oncolytic viral treatment induced an intratumoral mRNA transcription signature of tumor-specific immune responses in 83% (5/6) of canine glioma patients. Changes included mRNA signatures corresponding with interferon signaling, lymphoid and myeloid cell activation, recruitment, and T and B cell immunity. Multiplexed protein analysis identified a subset of oligodendroglioma subjects with increased concentrations of IL-2, IL-7, IL-6, IL-10, IL-15, TNFα, GM-CSF between 14 and 28 days after treatment, with evidence of CD4+ T cell activation and modulation of IL-4 and IFNγ production in CD4+ and CD8+ T cells isolated from peripheral blood.
Conclusion: These findings indicate that M032 modulates the tumor-immune microenvironment in the canine glioma model.
Keywords: M032; NanoString; Oncolytic herpes virus; canine glioma; large animal model; tumor microenvironment.
Conflict of interest statement
Conflicts of interest Markert JM and Gillespie GY are founders of and own stock and stock options (< 8% interest) in Aettis Inc., a biotech company developing other oHSVs that are not the subject of this current investigation. Gillespie GY currently serves as one of five unpaid members of the Board of Directors for Aettis Inc. Markert JM and Gillespie GY are founders of and own stock and stock options (< 25%) in Treovir, LLC, which has licensed G207 HSV that is not the subject of the current investigation. Gillespie GY has served as a paid advisor to the Program Project (Brigham and Women’s Hospital, Boston, MA) that seeks to find improved methods for the application of distinct oHSV to treat localized and metastatic cancers. This is generally, but not specifically, related to the subject matter of this investigation. Markert JM also holds intellectual property in another oHSV not the subject of this current investigation that has been licensed by Mustang Biotech Inc., and reports being an equity owner in Catherex (< 8%), which underwent a structured buyout by Amgen and no longer exists; and has served as a consultant for Imugene.
Figures

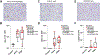




References
-
- Weller M, Wick W, Aldape K, et al. Glioma. Nat Rev Dis Primers 2015;1:15017. DOI PubMed - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359:492–507. DOI PubMed - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. DOI PubMed - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
