Aprotinin treatment against SARS-CoV-2: A randomized phase III study to evaluate the safety and efficacy of a pan-protease inhibitor for moderate COVID-19
- PMID: 35342931
- PMCID: PMC9111659
- DOI: 10.1111/eci.13776
Aprotinin treatment against SARS-CoV-2: A randomized phase III study to evaluate the safety and efficacy of a pan-protease inhibitor for moderate COVID-19
Abstract
Background: SARS-CoV-2 virus requires host proteases to cleave its spike protein to bind to its ACE2 target through a two-step furin-mediated entry mechanism. Aprotinin is a broad-spectrum protease inhibitor that has been employed as antiviral drug for other human respiratory viruses. Also, it has important anti-inflammatory properties for inhibiting the innate immunity contact system.
Methods: This was a multicentre, double-blind, randomized trial performed in four Spanish hospitals comparing standard treatment versus standard treatment + aprotinin for patients with COVID-19 between 20 May 2020 and 20 October 2021. The primary efficacy outcomes were length of hospital stay and ICU admission. The secondary endpoints were each of the primary efficacy outcomes and a composite of oxygen therapy, analytical parameters and death. Safety outcomes included adverse reactions to treatment during a 30-day follow-up period. Treatment was given for 11 days or till discharge.
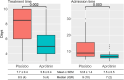
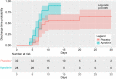
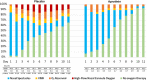
Results: With almost identical analytical profiles, significant differences were observed in treatment time, which was 2 days lower in the aprotinin group (p = .002), and length of hospital admission, which was 5 days shorter in the aprotinin group (p = .003). The incidence of discharge was 2.19 times higher (HR: 2.188 [1.182-4.047]) in the aprotinin group than in the placebo group (p = .013). In addition, the aprotinin-treated group required less oxygen therapy and had no adverse reactions or side effects.
Conclusion: Inhaled aprotinin may improve standard treatment and clinical outcomes in hospitalized patients with COVID-19, resulting in a shorter treatment time and hospitalization compared with the placebo group. The administration of aprotinin was safe.
Keywords: COVID-19; SARS-CoV-2; antivirus; aprotinin; clinical trial; inhalation therapy; pneumonia; protease inhibitor.
© 2022 The Authors. European Journal of Clinical Investigation published by John Wiley & Sons Ltd on behalf of Stichting European Society for Clinical Investigation Journal Foundation.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
References
-
- Levin AT, Hanage WP, Owusu‐Boaitey N, Cochran KB, Walsh SP, Meyerowitz‐Katz G. Assessing the age specificity of infection fatality rates for COVID‐19: systematic review, meta‐analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123‐1138. doi:10.1007/s10654-020-00698-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

