White paper: Oncofertility in pediatric patients with Wilms tumor
- PMID: 35342935
- PMCID: PMC9541948
- DOI: 10.1002/ijc.34006
White paper: Oncofertility in pediatric patients with Wilms tumor
Abstract
The survival of childhood Wilms tumor is currently around 90%, with many survivors reaching reproductive age. Chemotherapy and radiotherapy are established risk factors for gonadal damage and are used in both COG and SIOP Wilms tumor treatment protocols. The risk of infertility in Wilms tumor patients is low but increases with intensification of treatment including the use of alkylating agents, whole abdominal radiation or radiotherapy to the pelvis. Both COG and SIOP protocols aim to limit the use of gonadotoxic treatment, but unfortunately this cannot be avoided in all patients. Infertility is considered one of the most important late effects of childhood cancer treatment by patients and their families. Thus, timely discussion of gonadal damage risk and fertility preservation options is important. Additionally, irrespective of the choice for preservation, consultation with a fertility preservation (FP) team is associated with decreased patient and family regret and better quality of life. Current guidelines recommend early discussion of the impact of therapy on potential fertility. Since most patients with Wilms tumors are prepubertal, potential FP methods for this group are still considered experimental. There are no proven methods for FP for prepubertal males (testicular biopsy for cryopreservation is experimental), and there is just a single option for prepubertal females (ovarian tissue cryopreservation), posing both technical and ethical challenges. Identification of genetic markers of susceptibility to gonadotoxic therapy may help to stratify patient risk of gonadal damage and identify patients most likely to benefit from FP methods.
Keywords: Wilms tumor; fertility preservation; gonadal damage; pediatric cancer.
© 2022 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
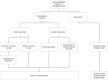
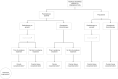
References
-
- de Kraker J, Graf N, van Tinteren H, et al. Reduction of postoperative chemotherapy in children with stage I intermediate‐risk and anaplastic Wilms' tumour (SIOP 93‐01 trial): a randomised controlled trial. Lancet. 2004;364(9441):1229‐1235. - PubMed
-
- Graf N, van Tinteren H, Bergeron C, et al. Characteristics and outcome of stage II and III non‐anaplastic Wilms' tumour treated according to the SIOP trial and study 93‐01. Eur J Cancer. 2012;48(17):3240‐3248. - PubMed
-
- Pritchard‐Jones K, Moroz V, Vujanic G, et al. Treatment and outcome of Wilms' tumour patients: an analysis of all cases registered in the UKW3 trial. Ann Oncol. 2012;23(9):2457‐2463. - PubMed
-
- Weirich A, Ludwig R, Graf N, et al. Survival in nephroblastoma treated according to the trial and study SIOP‐9/GPOH with respect to relapse and morbidity. Ann Oncol. 2004;15(5):808‐820. - PubMed

