A high-resolution dose calculation engine for X-ray microbeams radiation therapy
- PMID: 35342953
- PMCID: PMC9322281
- DOI: 10.1002/mp.15637
A high-resolution dose calculation engine for X-ray microbeams radiation therapy
Abstract
Background: Microbeam radiation therapy (MRT) is a treatment modality based on spatial fractionation of synchrotron generated X-rays into parallel, high dose, microbeams of a few microns width. MRT is still an underdevelopment radiosurgery technique for which, promising preclinical results on brain tumors and epilepsy encourages its clinical transfer.
Purpose: A safe clinical transfer of MRT needs a specific treatment planning system (TPS) that provides accurate dose calculations in human patients, taking into account the MRT beam's properties (high-dose gradients, spatial fractionation, polarization effects). So far, the most advanced MRT TPS, based on a hybrid dose calculation algorithm, is limited to a macroscopic rendering of the dose and does not account for the complex dose distribution inherent to MRT if delivered as conformal irradiations with multiple incidences. For overcoming these limitations, a multi-scale full Monte-Carlo calculation engine called penMRT has been developed and benchmarked against two general-purpose Monte Carlo (MC) codes: penmain based on PENELOPE and Gate based on Geant4.
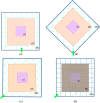
Methods: PenMRT, is based on the PENELOPE (2018) MC code, modified to take into account the voxelized geometry of the patients (computed tomography [CT]-scans) and is offering an adaptive micrometric dose calculation grid independent of the CT size, location, and orientation. The implementation of the dynamic memory allocation in penMRT, makes the simulations feasible within a huge number of dose scoring bins. The possibility of using a source replication approach to simulate arrays of microbeams, and the parallelization using OpenMPI have been added to penMRT in order to increase the calculation speed for clinical usages. This engine can be implemented in a TPS as a dose calculation core.
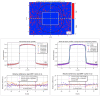
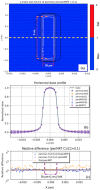
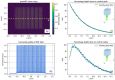
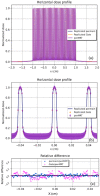
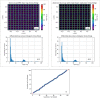
Results: The performance tests highlight the reliability of penMRT to be used for complex irradiation conditions in MRT. The benchmarking against a standard PENELOPE code did not show any significant difference for calculations in centimetric beams, for a single microbeam and for a microbeam array. The comparisons between penMRT and Gate as an independent MC code did not show any difference in the beam paths, whereas, in valley regions, relative differences between the two codes rank from 1% to 7.5% which are probably due to the differences in physics lists that are used in these two codes. The reliability of the source replication approach has also been tested and validated with an underestimation of no more than 0.6% in low-dose areas.
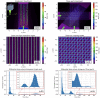
Conclusions: Good agreements (a relative difference between 0% and 8%) were found when comparing calculated peak to valley dose ratio values using penMRT, for irradiations with a full microbeam array, with calculated values in the literature. The high-resolution calculated dose maps obtained with penMRT are used to extract differential and cumulative dose-volume histograms (DVHs) and analyze treatment plans with much finer metrics regarding the irradiation complexity. To our knowledge, these are the first high-resolution dose maps and associated DVHs ever obtained for cross-fired microbeams irradiation, which is bringing a significant added value to the field of treatment planning in spatially fractionated radiation therapy.
Keywords: Monte Carlo method; dose calculation engine; medium energy X-rays; microbeam radiation therapy; micrometric dose calculation grids; synchrotron radiation.
© 2022 The Authors. Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

