Determining Losses in Jet Injection Subcutaneous Insulin Delivery: A Model-Based Approach
- PMID: 35343255
- PMCID: PMC10347999
- DOI: 10.1177/19322968221085032
Determining Losses in Jet Injection Subcutaneous Insulin Delivery: A Model-Based Approach
Abstract
Objective: Accurate, safe glycemic management requires reliable delivery of insulin doses. Insulin can be delivered subcutaneously for action over a longer period of time. Needle-free jet injectors provide subcutaneous (SC) delivery without requiring needle use, but the volume of insulin absorbed varies due to losses associated with the delivery method. This study employs model-based methods to determine the expected proportion of active insulin present from a needle-free SC dose.
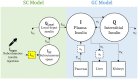
Methods: Insulin, C-peptide, and glucose assay data from a frequently sampled insulin-modified oral glucose tolerance test trial with 2U SC insulin delivery, paired with a well-validated metabolic model, predict metabolic outcomes for N = 7 healthy adults. Subject-specific nonlinear hepatic clearance profiles are modeled over time using third-order basis splines with knots located at assay times. Hepatic clearance profiles are constrained within a physiological rate of change, and relative to plasma glucose profiles. Insulin loss proportions yielding optimal insulin predictions are then identified, quantifying delivery losses.
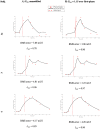
Results: Optimal parameter identification suggests losses of up to 22% of the nominal 2U SC dose. The degree of loss varies between subjects and between trials on the same subject. Insulin fit accuracy improves where loss greater than 5% is identified, relative to where delivery loss is not modeled.
Conclusions: Modeling shows needle-free SC jet injection of a nominal dose of insulin does not necessarily provide metabolic action equivalent to total dose, and this availability significantly varies between trials. By quantifying and accounting for variability of jet injection insulin doses, better glycemic management outcomes using SC jet injection may be achieved.
Keywords: OGTT; hepatic clearance; identification; identification error; insulin kinetics; insulin modified; jet injection; subcutaneous insulin.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Task Force on Jet Injectors, Council on Youth. Position statement on jet injectors. Diabetes Care. 1988;11:2. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

