Long-term Continuous Glucose Monitor Use in Very Young Children With Type 1 Diabetes: One-Year Results From the SENCE Study
- PMID: 35343269
- PMCID: PMC10348002
- DOI: 10.1177/19322968221084667
Long-term Continuous Glucose Monitor Use in Very Young Children With Type 1 Diabetes: One-Year Results From the SENCE Study
Abstract
Objectives: Achieving optimal glycemic outcomes in young children with type 1 diabetes (T1D) is challenging. This study examined the durability of continuous glucose monitoring (CGM) coupled with a family behavioral intervention (FBI) to improve glycemia.
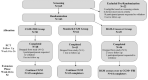
Study design: This one-year study included an initial 26-week randomized controlled trial of CGM with FBI (CGM+FBI) and CGM alone (Standard-CGM) compared with blood glucose monitoring (BGM), followed by a 26-week extension phase wherein the BGM Group received the CGM+FBI (BGM-Crossover) and both original CGM groups continued this technology.
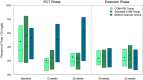
Results: Time in range (70-180 mg/dL) did not improve with CGM use (CGM+FBI: baseline 37%, 52 weeks 41%; Standard-CGM: baseline 41%, 52 weeks 44%; BGM-Crossover: 26 weeks 38%, 52 weeks 40%). All three groups sustained decreases in hypoglycemia (<70 mg/dL) with CGM use (CGM+FBI: baseline 3.4%, 52 weeks 2.0%; Standard-CGM: baseline 4.1%, 52 weeks 2.1%; BGM-Crossover: 26 weeks 4.5%, 52 weeks 1.7%, P-values <.001). Hemoglobin A1c was unchanged with CGM use (CGM+FBI: baseline 8.3%, 52 weeks 8.2%; Standard-CGM: baseline 8.2%, 52 weeks 8.0%; BGM-Crossover: 26 weeks 8.1%, 52 weeks 8.3%). Sensor use remained high (52-week study visit: CGM+FBI 91%, Standard-CGM 92%, BGM-Crossover 88%).
Conclusion: Over 12 months young children with T1D using newer CGM technology sustained reductions in hypoglycemia and, in contrast to prior studies, persistently wore CGM. However, pervasive hyperglycemia remained unmitigated. This indicates an urgent need for further advances in diabetes technology, behavioral support, and diabetes management educational approaches to optimize glycemia in young children.
Keywords: behavioral intervention; continuous glucose monitoring; glycemic control; hypoglycemia; young children.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.A.V.N., L.G.K., L.A.D., K.M.M., A.A.-O., P.C., S.D.C., K.R.H., M.E.H., B.J.A., J.C.K., S.A.M., B.M.N., W.V.T., K.M.W., K.A.W., S.W., and J.C.W. have no disclosures to report. L.M.L. reports grants and personal fees from Dexcom, outside the submitted work. R.P.W. reports grants from Dexcom, Eli Lilly, and Tandem Diabetes Care and personal fees from Dompe and Tandem Diabetes Care outside the submitted work. S.M.W. reports personal fees from Roche Diagnostics and Boehringer Ingelheim, outside the submitted work. D.J.D. reports research support from Insulet and personal fees from Dexcom outside the submitted work.
Figures

References
-
- Sundberg F, Forsander G. Detection and treatment efficacy of hypoglycemic events in the everyday life of children younger than 7 yr. Pediatr Diabetes. 2014;15:34-40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

