Functional proteomics of patient derived head and neck squamous cell carcinoma cells reveal novel applications of trametinib
- PMID: 35343367
- PMCID: PMC8966983
- DOI: 10.1080/15384047.2022.2055420
Functional proteomics of patient derived head and neck squamous cell carcinoma cells reveal novel applications of trametinib
Abstract
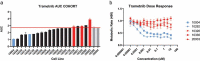
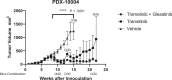
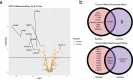
In this study, we report a differential response of mitogen-activated protein kinase-kinase (MEK) inhibitor trametinib in 20 head and neck squamous cell carcinoma (HNSCC) patients' tumor-derived cell cultures. Relatively sensitive and resistant cases to trametinib were identified using high throughput metabolic assays and validated in extended dose response studies in vitro. High throughput metabolic assays exploring combination therapies with trametinib were subjected to synergy models and maximal synergistic dose analyses. These yielded several candidates, including axtinib, GDC-0032, GSK-690693, and SGX-523. The combination regimen of trametinib and AXL/MET/VEGFR inhibitor glesatinib showed initial efficacy both in vitro and in vivo (92% reduction in tumor volume). Sensitivity was validated in vivo in a patient-derived xenograft (PDX) model in which trametinib as a single agent effected reduction in tumor volume up to 72%. Reverse Phase Protein Arrays (RPPA) demonstrated differentially expressed proteins and phosphoproteins upon trametinib treatment. Furthermore, resistant cell lines showed a compensatory mechanism via increases in MAPK and non-MAPK pathway proteins that may represent targets for future combination regimens. Intrinsic-targeted options have potential to address paucity of medical treatment options for HNSCC cancer patients, enhance response to extrinsic targeted agents, and/or reduce morbidity as neoadjuvant to surgical treatments.
Keywords: Cancer; HNSCC; MAPK; PDX; Trametinib.
Conflict of interest statement
J.W.T. received research support from Agios, Aptose, Array, AstraZeneca, Constellation, Genentech, Gilead, Incyte, Janssen, Petra, Seattle Genetics, Syros, Takeda, and Tolero
Figures

References
-
- Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar AK, et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. JCO. 2014;32(27):2940–2950. doi: 10.1200/JCO.2013.53.5633. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
