The role of FYCO1-dependent autophagy in lens fiber cell differentiation
- PMID: 35343376
- PMCID: PMC9397473
- DOI: 10.1080/15548627.2022.2025570
The role of FYCO1-dependent autophagy in lens fiber cell differentiation
Abstract
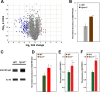
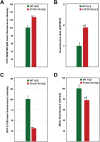
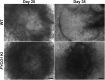
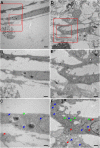
FYCO1 (FYVE and coiled-coil domain containing 1) is an adaptor protein, expressed ubiquitously and required for microtubule-dependent, plus-end-directed transport of macroautophagic/autophagic vesicles. We have previously shown that loss-of-function mutations in FYCO1 cause cataracts with no other ocular and/or extra-ocular phenotype. Here, we show fyco1 homozygous knockout (fyco1-/-) mice recapitulate the cataract phenotype consistent with a critical role of FYCO1 and autophagy in lens morphogenesis. Transcriptome coupled with proteome and metabolome profiling identified many autophagy-associated genes, proteins, and lipids respectively perturbed in fyco1-/- mice lenses. Flow cytometry of FYCO1 (c.2206C>T) knock-in (KI) human lens epithelial cells revealed a decrease in autophagic flux and autophagic vesicles resulting from the loss of FYCO1. Transmission electron microscopy showed cellular organelles accumulated in FYCO1 (c.2206C>T) KI lens-like organoid structures and in fyco1-/- mice lenses. In summary, our data confirm the loss of FYCO1 function results in a diminished autophagic flux, impaired organelle removal, and cataractogenesis.Abbreviations: CC: congenital cataracts; DE: differentially expressed; ER: endoplasmic reticulum; FYCO1: FYVE and coiled-coil domain containing 1; hESC: human embryonic stem cell; KI: knock-in; OFZ: organelle-free zone; qRT-PCR: quantitative real-time PCR; PE: phosphatidylethanolamine; RNA-Seq: RNA sequencing; SD: standard deviation; sgRNA: single guide RNA; shRNA: shorthairpin RNA; TEM: transmission electron microscopy; WT: wild type.
Keywords: Autophagy; cataracts; lens fiber cells; organelle removal; organelle-free zone.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Jean D, Ewan K, Gruss P.. Molecular regulators involved in vertebrate eye development. Mech Dev. 1998;76(1–2):3–18. - PubMed
-
- McAvoy JW, Chamberlain CG, de Iongh RU, et al. Lens development. Eye (Lond). 1999;13(Pt 3b):425–437. - PubMed
-
- Zandy AJ, Bassnett S. Proteolytic mechanisms underlying mitochondrial degradation in the ocular lens. Invest Ophthalmol Vis Sci. 2007;48(1):293–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
