Current methods to analyze lysosome morphology, positioning, motility and function
- PMID: 35343629
- PMCID: PMC9323414
- DOI: 10.1111/tra.12839
Current methods to analyze lysosome morphology, positioning, motility and function
Abstract
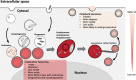
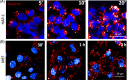
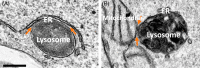
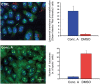
Since the discovery of lysosomes more than 70 years ago, much has been learned about the functions of these organelles. Lysosomes were regarded as exclusively degradative organelles, but more recent research has shown that they play essential roles in several other cellular functions, such as nutrient sensing, intracellular signalling and metabolism. Methodological advances played a key part in generating our current knowledge about the biology of this multifaceted organelle. In this review, we cover current methods used to analyze lysosome morphology, positioning, motility and function. We highlight the principles behind these methods, the methodological strategies and their advantages and limitations. To extract accurate information and avoid misinterpretations, we discuss the best strategies to identify lysosomes and assess their characteristics and functions. With this review, we aim to stimulate an increase in the quantity and quality of research on lysosomes and further ground-breaking discoveries on an organelle that continues to surprise and excite cell biologists.
Keywords: TFEB; endolysosomes; lysosomal storage diseases; lysosome biogenesis; lysosome exocytosis; lysosome-related organelles; lysosomes; mTOR; membrane contact sites.
© 2022 The Authors. Traffic published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures





References
-
- De Duve C. The separation and characterization of subcellular particles. Harvey Lect. 1965;59:49‐87. - PubMed
-
- Castro‐Obregon S. The discovery of lysosomes and autophagy. Nat Educ. 2010;3:49.
-
- Straus W. Isolation and biochemical properties of droplets from the cells of rat kidney. J Biol Chem. 1954;207:745‐755. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

