Quality of life in patients with locally advanced head and neck squamous cell carcinoma undergoing concurrent chemoradiation with once-a-week versus once-every-3-weeks cisplatin
- PMID: 35343648
- PMCID: PMC9636500
- DOI: 10.1002/cam4.4715
Quality of life in patients with locally advanced head and neck squamous cell carcinoma undergoing concurrent chemoradiation with once-a-week versus once-every-3-weeks cisplatin
Abstract
Introduction: This trial was conducted to compare the efficacy of low dose once-a-week cisplatin and once-every-3-weeks cisplatin with radiation in locally advanced head and neck squamous cell carcinoma (LAHNSCC). The current analysis focuses on the quality of life (QoL) of patients in this trial.
Methods: In this phase III randomized trial, patients with nonmetastatic LAHNSCC were randomized to receive cisplatin 30 mg/m2 once-a-week or 100 mg/m2 once every- 3-weeks concurrently with radiotherapy. The primary endpoint was locoregional control. QoL was a key secondary endpoint. QoL was assessed using EORTC QLQ-C30 and QLQ-H&N35. QoL data were assessed at baseline, days 22 and 43 during treatment; and at 6, 12, 24 months. The linear mixed-effects model was used for longitudinal analysis of QoL to determine the impact of treatment (arm) and time on QoL.
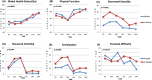
Results: Three hundred patients were enrolled, data of 150 patients with available baseline QoL were analyzed. There was no significant difference in the global health status/QoL of the two treatment arms (p = 0.8664). There was no significant difference in the longitudinal QoL scores between the two treatment arms in all scales except constipation (p = 0.0096), less sexuality (p = 0.0002,), and financial difficulty (p = 0.0219). There was a worsening of the QoL scores in all scales in both arms during treatment, which improved after treatment completion in most scales.
Conclusion: The use of once-every-3-weeks cisplatin did not adversely impact QoL as compared to once-a-week cisplatin in combination with radiotherapy in LAHNSCC.
Keywords: chemoradiation; cisplatin; head and neck cancer; quality of life.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Vanita Noronha reports grants from Dr. Reddy's Laboratories, Amgen, and Sanofi, outside of the submitted work. Kumar Prabhash reports grants from Tata Memorial Center Research Administration Council, the Indian Cooperative Oncology Network, and Glenmark Pharmaceuticals, during the conduct of the study; grants from Dr. Reddy's Laboratories, Fresenius Kabi India, and Roche Holding, outside of the submitted work. All other authors declare no conflict of interest.
Figures
References
-
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945‐1952. - PubMed
-
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high‐risk squamous‐cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937‐1944. - PubMed
-
- Goyal G, Patil VM, Noronha V, et al. Once‐a‐week versus once‐every‐3‐weeks cisplatin in patients receiving chemoradiation for locally advanced head‐and‐neck cancer: a survey of practice in India. Cancer Res Stat Treat. 2018;1:63‐67.
-
- Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systemic literature review. Radiother Oncol. 2003;66:253‐262. - PubMed
-
- Langendijk JA, Doornaert P, Verdonck‐de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment‐related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770‐3776. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

