Quantitative Proteomics Reveals Metabolic Reprogramming in Host Cells Induced by Trophozoites and Intermediate Subunit of Gal/GalNAc Lectins from Entamoeba histolytica
- PMID: 35343800
- PMCID: PMC9040881
- DOI: 10.1128/msystems.01353-21
Quantitative Proteomics Reveals Metabolic Reprogramming in Host Cells Induced by Trophozoites and Intermediate Subunit of Gal/GalNAc Lectins from Entamoeba histolytica
Abstract
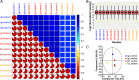
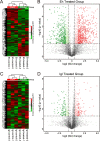
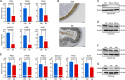
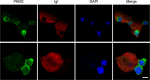
Entamoeba histolytica is an intestinal protozoan parasite with remarkable ability to kill and phagocytose host cells, causing amoebic colitis and extraintestinal abscesses. The intermediate subunit (Igl) of galactose (Gal)- and N-acetyl-d-galactosamine (GalNAc)-specific lectins is considered an important surface antigen involved in the pathogenesis of E. histolytica. Here, we applied mass spectrometry-based quantitative proteomics technology to analyze the protein expression profile changes occurring in host Caco2 cells incubated with E. histolytica trophozoites or stimulated by purified native Igl protein. The expression levels of 1,490 and 489 proteins were significantly altered in the E. histolytica-treated and Igl-treated groups, respectively, among 6,875 proteins totally identified. Intriguingly, central carbon metabolism of host cells was suppressed in both E. histolytica-treated and Igl-treated groups, with evidence of decreased expression levels of several key enzymes, including pyruvate kinase muscle type 2, presenting a Warburg-like effect in host cells. Besides, Igl had potential physical interactions with central carbon metabolism enzymes and the proteolytic degradation family members proteasome subunit alpha and beta, which may be responsible for the degradation of key enzymes in carbon metabolism. These results provided a novel perspective on the pathogenic mechanism of E. histolytica and compelling evidence supporting the important role of Igl in the virulence of E. histolytica. IMPORTANCE Metabolic reprogramming is considered a hallmark of some infectious diseases. However, in amoebiasis, a neglected tropical disease caused by protozoan parasite E. histolytica, metabolic changes in host cells have yet to be proven. In this study, advanced data-independent acquisition mass spectrometry-based quantitative proteomics was applied to investigate the overall host cellular metabolic changes as high-throughput proteomics could measure molecular changes in a cell or tissue with high efficiency. Enrichment analysis of differentially expressed proteins showed biological processes and cellular pathways related to amoeba infection and Igl cytotoxicity. Specifically, central carbon metabolism of host cells was dramatically suppressed in both E. histolytica-treated and Igl-treated groups, indicating the occurrence of a Warburg-like effect induced by trophozoites or Igl from E. histolytica. Distinct differences in ubiquitin-mediated proteolysis, rapamycin (mTOR) signaling pathway, autophagy, endocytosis, and tight junctions provided novel perspectives on the pathogenic mechanism of E. histolytica.
Keywords: Entamoeba histolytica; Gal/GalNAc lectins; metabolic reprogramming; pyruvate kinase; quantitative proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Identification and characterization of a carbohydrate recognition domain-like region in Entamoeba histolytica Gal/GalNAc lectin intermediate subunit.Microbiol Spectr. 2024 Nov 5;12(11):e0053824. doi: 10.1128/spectrum.00538-24. Epub 2024 Oct 4. Microbiol Spectr. 2024. PMID: 39365081 Free PMC article.
-
Multi-omics insights into the metabolic reprogramming of host cells triggered by Entamoeba histolytica Gal/GalNAc lectin intermediate subunit.Microbiol Spectr. 2025 Aug 20:e0038125. doi: 10.1128/spectrum.00381-25. Online ahead of print. Microbiol Spectr. 2025. PMID: 40833094
-
Evaluation of the C-Terminal Fragment of Entamoeba histolytica Gal/GalNAc Lectin Intermediate Subunit as a Vaccine Candidate against Amebic Liver Abscess.PLoS Negl Trop Dis. 2016 Jan 29;10(1):e0004419. doi: 10.1371/journal.pntd.0004419. eCollection 2016 Jan. PLoS Negl Trop Dis. 2016. PMID: 26824828 Free PMC article.
-
Recent developments in amoebiasis:the Gal/GalNAc lectins of Entamoeba histolytica and Entamoeba dispar.Microbes Infect. 2000 Nov;2(14):1775-83. doi: 10.1016/s1286-4579(00)01330-7. Microbes Infect. 2000. PMID: 11137050 Review.
-
Entamoeba histolytica: from adherence to enteropathy.J Infect Dis. 1989 Mar;159(3):420-9. doi: 10.1093/infdis/159.3.420. J Infect Dis. 1989. PMID: 2536786 Review.
References
-
- Royer TL, Petri WA. 2014. Waterborne parasites | Entamoeba, p 782–786. In Batt C, Tortorello ML (ed), Encyclopedia of food microbiology, 2nd ed. Elsevier, Cambridge, MA.
-
- El-Dib NA, Khater MM. 2020. Entamoeba. In Reference module in biomedical sciences. Elsevier, Amsterdam, Netherlands. doi:10.1016/B978-0-12-818731-9.00024-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

