Salivary gland function, development, and regeneration
- PMID: 35343828
- PMCID: PMC9126227
- DOI: 10.1152/physrev.00015.2021
Salivary gland function, development, and regeneration
Abstract
Salivary glands produce and secrete saliva, which is essential for maintaining oral health and overall health. Understanding both the unique structure and physiological function of salivary glands, as well as how they are affected by disease and injury, will direct the development of therapy to repair and regenerate them. Significant recent advances, particularly in the OMICS field, increase our understanding of how salivary glands develop at the cellular, molecular, and genetic levels: the signaling pathways involved, the dynamics of progenitor cell lineages in development, homeostasis, and regeneration, and the role of the extracellular matrix microenvironment. These provide a template for cell and gene therapies as well as bioengineering approaches to repair or regenerate salivary function.
Keywords: exocrine secretion; gene therapy; progenitor cell; salivary gland; xerostomia.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







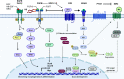
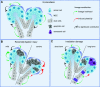

References
-
- Lan X, Chan JY, Pu JJ, Qiao W, Pang S, Yang WF, Wong KC, Kwong DL, Su YX. Saliva electrolyte analysis and xerostomia-related quality of life in nasopharyngeal carcinoma patients following intensity-modulated radiation therapy. Radiother Oncol 150: 97–103, 2020. doi: 10.1016/j.radonc.2020.06.016. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

