Association of Glucagon-Like Peptide-1 Receptor Agonist Use With Risk of Gallbladder and Biliary Diseases: A Systematic Review and Meta-analysis of Randomized Clinical Trials
- PMID: 35344001
- PMCID: PMC8961394
- DOI: 10.1001/jamainternmed.2022.0338
Association of Glucagon-Like Peptide-1 Receptor Agonist Use With Risk of Gallbladder and Biliary Diseases: A Systematic Review and Meta-analysis of Randomized Clinical Trials
Abstract
Importance: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have been widely recommended for glucose control and cardiovascular risk reduction in patients with type 2 diabetes, and more recently, for weight loss. However, the associations of GLP-1 RAs with gallbladder or biliary diseases are controversial.
Objective: To evaluate the association of GLP-1 RA treatment with gallbladder and biliary diseases and to explore risk factors for these associations.
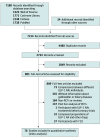
Data sources: MEDLINE/PubMed, EMBASE, Web of Science, and Cochrane Library (inception to June 30, 2021), websites of clinical trial registries (July 10, 2021), and reference lists. There were no language restrictions.
Study selection: Randomized clinical trials (RCTs) comparing the use of GLP-1 RA drugs with placebo or with non-GLP-1 RA drugs in adults.
Data extraction and synthesis: Two reviewers independently extracted data according to the PRISMA recommendations and assessed the quality of each study with the Cochrane Collaboration risk-of-bias tool. Pooled relative risks (RRs) were calculated using random or fixed-effects models, as appropriate. The quality of evidence for each outcome was assessed using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) framework.
Main outcomes and measures: The primary outcome was the composite of gallbladder or biliary diseases. Secondary outcomes were biliary diseases, biliary cancer, cholecystectomy, cholecystitis, and cholelithiasis. Data analyses were performed from August 5, 2021, to September 3, 2021.
Results: A total of 76 RCTs involving 103 371 patients (mean [SD] age, 57.8 (6.2) years; 41 868 [40.5%] women) were included. Among all included trials, randomization to GLP-1 RA treatment was associated with increased risks of gallbladder or biliary diseases (RR, 1.37; 95% CI, 1.23-1.52); specifically, cholelithiasis (RR, 1.27; 95% CI, 1.10-1.47), cholecystitis (RR, 1.36; 95% CI, 1.14-1.62), and biliary disease (RR, 1.55; 95% CI, 1.08-2.22). Use of GLP-1 RAs was also associated with increased risk of gallbladder or biliary diseases in trials for weight loss (n = 13; RR, 2.29; 95% CI, 1.64-3.18) and for type 2 diabetes or other diseases (n = 63; RR, 1.27; 95% CI, 1.14-1.43; P <.001 for interaction). Among all included trials, GLP-1 RA use was associated with higher risks of gallbladder or biliary diseases at higher doses (RR, 1.56; 95% CI, 1.36-1.78) compared with lower doses (RR, 0.99; 95% CI, 0.73-1.33; P = .006 for interaction) and with longer duration of use (RR, 1.40; 95% CI, 1.26-1.56) compared with shorter duration (RR, 0.79; 95% CI, 0.48-1.31; P = .03 for interaction).
Conclusions and relevance: This systematic review and meta-analysis of RCTs found that use of GLP-1 RAs was associated with increased risk of gallbladder or biliary diseases, especially when used at higher doses, for longer durations, and for weight loss.
Trial registration: PROSPERO Identifier: CRD42021271599.
Conflict of interest statement
Figures


Comment in
-
Glucagon-Like Peptide-1 Receptor Agonists-How Safe Are They?JAMA Intern Med. 2022 May 1;182(5):520-521. doi: 10.1001/jamainternmed.2022.0335. JAMA Intern Med. 2022. PMID: 35344015 No abstract available.
References
-
- Wadden TA, Bailey TS, Billings LK, et al. ; STEP 3 Investigators . Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413. doi:10.1001/jama.2021.1831 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

