Cost-effectiveness of Aducanumab and Donanemab for Early Alzheimer Disease in the US
- PMID: 35344024
- PMCID: PMC8961406
- DOI: 10.1001/jamaneurol.2022.0315
Cost-effectiveness of Aducanumab and Donanemab for Early Alzheimer Disease in the US
Abstract
Importance: Several anti-amyloid monoclonal antibodies have been developed for slowing the progression of Alzheimer disease (AD). Among the furthest developed are aducanumab, which received accelerated approval from the US Food and Drug Administration in 2021, and donanemab, which is currently undergoing phase 3 trials. The cost-effectiveness of these treatments has not been established.
Objectives: To estimate the cost-effectiveness of aducanumab and donanemab relative to standard care for early AD in the US.
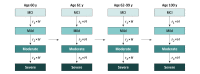
Design, setting, and participants: A decision analytic model was used to estimate the lifetime health and economic outcomes of adults with early AD, from US healthcare sector and societal perspectives. Simulated patients had a mean (SD) age of 75.2 (5.5) years; 65% had mild cognitive impairment and 35% had mild dementia. Analyses were conducted from April 6, 2021, to January 20, 2022.
Interventions: Standard care, aducanumab (selected inputs including disease progression hazard ratio [HR] of 0.89 [95% CI, 0.63-1.15], annual price of $28 000, and twice-yearly monitoring with magnetic resonance imaging [MRI] of the brain), or donanemab (selected inputs including disease progression HR of 0.68 [95% CI, 0.44-0.99], annual price of $28 000, and twice-yearly monitoring with MRI of the brain and amyloid positron emission tomography [PET] monitoring). Donanemab was switched to placebo after substantial amyloid reduction on PET imaging, which occurred in 27% of patients at 6 months and 55% of patients at 12 months.
Main outcomes and measures: Quality-adjusted life-years (QALYs); costs, in 2020 US dollars; incremental cost-effectiveness ratios (ICERs); and value-based prices, defined as the maximum price at which a treatment would be cost-effective given a cost-effectiveness threshold of ICER of $150 000/QALY.
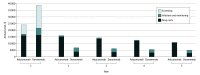
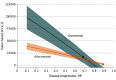
Results: Lifetime QALYs increased by 0.133 with aducanumab and 0.408 with donanemab. Total health care sector and societal costs increased by $130 100 and $127 800, respectively, with aducanumab and by $78 700 and $71 600, respectively, with donanemab, driven largely by drug costs ($119 000 for aducanumab and $44 600 for donanemab). Health care sector and societal ICERs relative to standard care were $981 000/QALY and $964 000/QALY, respectively, for aducanumab and $193 000/QALY and $176 000/QALY, respectively, for donanemab. In sensitivity analysis, aducanumab's value-based price remained less than $50 000/y, even when assuming a 90% reduction in disease progression. Donanemab's value-based price surpassed $50 000/y once its efficacy exceeded 50%.
Conclusions and relevance: These findings suggest that at current expected prices, neither aducanumab nor donanemab would be cost-effective for early AD in the US. Donanemab's dosing scheme, in which patients suspend treatment on achieving substantial amyloid reductions, may provide a rubric by which sufficiently effective anti-amyloid antibody treatments could be cost-effective even when priced comparably to other biologics.
Conflict of interest statement
Figures
References
-
- Budd Haeberlein S, von Hehn C, Tian Y, et al. . EMERGE and ENGAGE topline results: two phase 3 studies to evaluate aducanumab in patients with early Alzheimer’s disease. Paper presented at: 2019 Annual Clinical Trials on Alzheimer's Disease conference; December 5, 2019; San Diego, California.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

