Phase II Multi-institutional Clinical Trial Result of Concurrent Cetuximab and Nivolumab in Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma
- PMID: 35344035
- PMCID: PMC9167762
- DOI: 10.1158/1078-0432.CCR-21-3849
Phase II Multi-institutional Clinical Trial Result of Concurrent Cetuximab and Nivolumab in Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma
Abstract
Purpose: A phase II multi-institutional clinical trial was conducted to determine overall survival (OS) in patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) treated with a combination of cetuximab and nivolumab.
Patients and methods: Patients with R/M HNSCC were treated with cetuximab 500 mg/m2 i.v. on day 14 as a lead-in followed by cetuximab 500 mg/m2 i.v. and nivolumab 240 mg i.v. on day 1 and day 15 of each 28-day cycle. Expression of p16 and programmed cell death-ligand 1 (PD-L1) in archived tumors were determined. Tumor-tissue-modified human papillomavirus (TTMV) DNA was quantified in plasma.
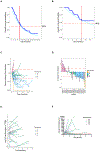
Results: Ninety-five patients were enrolled, and 88 patients were evaluable for OS with a median follow-up of 15.9 months. Median OS in the 45 patients who had prior therapy for R/M HNSCC (cohort A) was 11.4 months, with a 1 year OS 50% [90% confidence interval (CI), 0.43-0.57]. Median OS in the 43 patients who had no prior therapy (cohort B) was 20.2 months, with a 1-year OS 66% (90% CI, 0.59-0.71). In the combined cohorts, the p16-negative immunostaining was associated with higher response rate (RR; P = 0.02) but did not impact survival while higher PD-L1 combined positive score was associated with higher RR (P = 0.03) and longer OS (log-rank P = 0.04). In the p16-positive patients, lower median (1,230 copies/mL) TTMV DNA counts were associated with higher RR (P = 0.01) and longer OS compared with higher median (log-rank P = 0.05).
Conclusions: The combination of cetuximab and nivolumab is effective in patients with both previously treated and untreated R/M HNSCC and warrants further evaluation.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. - PubMed
-
- Maier H, Dietz A, Gewelke U, Heller WD, Weidauer H. Tobacco and alcohol and the risk of head and neck cancer. Clin Investig. 1992;70:320–7. - PubMed
-
- Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. - PubMed
-
- D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

