ER-mitochondria communication is involved in NLRP3 inflammasome activation under stress conditions in the innate immune system
- PMID: 35344105
- PMCID: PMC11072401
- DOI: 10.1007/s00018-022-04211-7
ER-mitochondria communication is involved in NLRP3 inflammasome activation under stress conditions in the innate immune system
Abstract
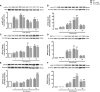
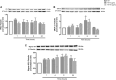
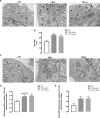
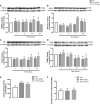
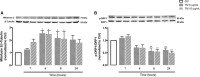
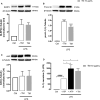
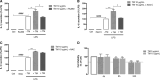
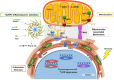
Endoplasmic reticulum (ER) stress and mitochondrial dysfunction, which are key events in the initiation and/or progression of several diseases, are correlated with alterations at ER-mitochondria contact sites, the so-called "Mitochondria-Associated Membranes" (MAMs). These intracellular structures are also implicated in NLRP3 inflammasome activation which is an important driver of sterile inflammation, however, the underlying molecular basis remains unclear. This work aimed to investigate the role of ER-mitochondria communication during ER stress-induced NLRP3 inflammasome activation in both peripheral and central innate immune systems, by using THP-1 human monocytes and BV2 microglia cells, respectively, as in vitro models. Markers of ER stress, mitochondrial dynamics and mass, as well as NLRP3 inflammasome activation were evaluated by Western Blot, IL-1β secretion was measured by ELISA, and ER-mitochondria contacts were quantified by transmission electron microscopy. Mitochondrial Ca2+ uptake and polarization were analyzed with fluorescent probes, and measurement of aconitase and SOD2 activities monitored mitochondrial ROS accumulation. ER stress was demonstrated to activate the NLRP3 inflammasome in both peripheral and central immune cells. Studies in monocytes indicate that ER stress-induced NLRP3 inflammasome activation occurs by a Ca2+-dependent and ROS-independent mechanism, which is coupled with upregulation of MAMs-resident chaperones, closer ER-mitochondria contacts, as well as mitochondrial depolarization and impaired dynamics. Moreover, enhanced ER stress-induced NLRP3 inflammasome activation in the immune system was found associated with pathological conditions since it was observed in monocytes derived from bipolar disorder (BD) patients, supporting a pro-inflammatory status in BD. In conclusion, by demonstrating that ER-mitochondria communication plays a key role in the response of the innate immune cells to ER stress, this work contributes to elucidate the molecular mechanisms underlying NLRP3 inflammasome activation under stress conditions, and to disclose novel potential therapeutic targets for diseases associated with sterile inflammation.
Keywords: Bipolar disorder (BD); Calcium; Endoplasmic reticulum (ER) stress; Mitochondria; Sterile inflammation; Unfolded protein response.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

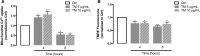


Similar articles
-
Mitochondria Fusion upon SERCA Inhibition Prevents Activation of the NLRP3 Inflammasome in Human Monocytes.Cells. 2022 Jan 27;11(3):433. doi: 10.3390/cells11030433. Cells. 2022. PMID: 35159243 Free PMC article.
-
Anthocyanins from Hibiscus syriacus L. Inhibit NLRP3 Inflammasome in BV2 Microglia Cells by Alleviating NF-κB- and ER Stress-Induced Ca2+ Accumulation and Mitochondrial ROS Production.Oxid Med Cell Longev. 2021 Feb 4;2021:1246491. doi: 10.1155/2021/1246491. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33613822 Free PMC article.
-
The Roles of Endoplasmic Reticulum in NLRP3 Inflammasome Activation.Cells. 2020 May 14;9(5):1219. doi: 10.3390/cells9051219. Cells. 2020. PMID: 32423023 Free PMC article. Review.
-
Augmented microglial endoplasmic reticulum-mitochondria contacts mediate depression-like behavior in mice induced by chronic social defeat stress.Nat Commun. 2024 Jun 18;15(1):5199. doi: 10.1038/s41467-024-49597-z. Nat Commun. 2024. PMID: 38890305 Free PMC article.
-
Emerging insights into the role of NLRP3 inflammasome and endoplasmic reticulum stress in renal diseases.Int Immunopharmacol. 2024 Jul 30;136:112342. doi: 10.1016/j.intimp.2024.112342. Epub 2024 May 30. Int Immunopharmacol. 2024. PMID: 38820956 Review.
Cited by
-
L-Proline Prevents Endoplasmic Reticulum Stress in Microglial Cells Exposed to L-azetidine-2-carboxylic Acid.Molecules. 2023 Jun 16;28(12):4808. doi: 10.3390/molecules28124808. Molecules. 2023. PMID: 37375363 Free PMC article.
-
cGAS-STING pathway in ischemia-reperfusion injury: a potential target to improve transplantation outcomes.Front Immunol. 2023 Sep 21;14:1231057. doi: 10.3389/fimmu.2023.1231057. eCollection 2023. Front Immunol. 2023. PMID: 37809088 Free PMC article. Review.
-
STING contributes to lipopolysaccharide-induced tubular cell inflammation and pyroptosis by activating endoplasmic reticulum stress in acute kidney injury.Cell Death Dis. 2024 Mar 14;15(3):217. doi: 10.1038/s41419-024-06600-1. Cell Death Dis. 2024. PMID: 38485717 Free PMC article.
-
Single-cell atlas reveals the immunosuppressive microenvironment and Treg cells landscapes in recurrent Glioblastoma.Cancer Gene Ther. 2024 May;31(5):790-801. doi: 10.1038/s41417-024-00740-4. Epub 2024 Mar 1. Cancer Gene Ther. 2024. PMID: 38429367
-
HMGB1 promotes mitochondrial transfer between hepatocellular carcinoma cells through RHOT1 and RAC1 under hypoxia.Cell Death Dis. 2024 Feb 20;15(2):155. doi: 10.1038/s41419-024-06536-6. Cell Death Dis. 2024. PMID: 38378644 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

