Fine-tuning of epithelial taste bud organoid to promote functional recapitulation of taste reactivity
- PMID: 35344108
- PMCID: PMC8958342
- DOI: 10.1007/s00018-022-04242-0
Fine-tuning of epithelial taste bud organoid to promote functional recapitulation of taste reactivity
Abstract
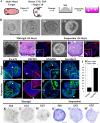
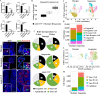
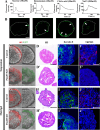
Taste stem/progenitor cells from posterior mouse tongues have been used to generate taste bud organoids. However, the inaccessible location of taste receptor cells is observed in conventional organoids. In this study, we established a suspension-culture method to fine-tune taste bud organoids by apicobasal polarity alteration to form the accessible localization of taste receptor cells. Compared to conventional Matrigel-embedded organoids, suspension-cultured organoids showed comparable differentiation and renewal rates to those of taste buds in vivo and exhibited functional taste receptor cells and cycling progenitor cells. Accessible taste receptor cells enabled the direct application of calcium imaging to evaluate the taste response. Moreover, suspension-cultured organoids can be genetically altered. Suspension-cultured taste bud organoids harmoniously integrated with the recipient lingual epithelium, maintaining the taste receptor cells and gustatory innervation capacity. We propose that suspension-cultured organoids may provide an efficient model for taste research, including taste bud development, regeneration, and transplantation.
Keywords: Apicobasal polarity; Calcium imaging; Genetic alteration; Single-cell RNA sequencing; Suspension-culture; Taste bud organoids; Taste receptor cells; Transplantation.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

Similar articles
-
Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo.Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16401-6. doi: 10.1073/pnas.1409064111. Epub 2014 Nov 3. Proc Natl Acad Sci U S A. 2014. PMID: 25368147 Free PMC article.
-
Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid.Sci Rep. 2015 Nov 24;5:17185. doi: 10.1038/srep17185. Sci Rep. 2015. PMID: 26597788 Free PMC article.
-
Transcellular labeling by DiI demonstrates the glossopharyngeal innervation of taste buds in the lingual epithelium of the axolotl.J Comp Neurol. 1993 May 1;331(1):122-33. doi: 10.1002/cne.903310108. J Comp Neurol. 1993. PMID: 8320345
-
Preparation and application of taste bud organoids in biomedicine towards chemical sensation mechanisms.Biotechnol Bioeng. 2022 Aug;119(8):2015-2030. doi: 10.1002/bit.28109. Epub 2022 May 2. Biotechnol Bioeng. 2022. PMID: 35441364 Review.
-
Building sensory receptors on the tongue.J Neurocytol. 2004 Dec;33(6):631-46. doi: 10.1007/s11068-005-3332-0. Epub 2005 Oct 11. J Neurocytol. 2004. PMID: 16217619 Review.
Cited by
-
Revolutionising oral organoids with artificial intelligence.Biomater Transl. 2024 Nov 15;5(4):372-389. doi: 10.12336/biomatertransl.2024.04.004. eCollection 2024. Biomater Transl. 2024. PMID: 39872928 Free PMC article. Review.
-
Physiology of the tongue with emphasis on taste transduction.Physiol Rev. 2023 Apr 1;103(2):1193-1246. doi: 10.1152/physrev.00012.2022. Epub 2022 Nov 24. Physiol Rev. 2023. PMID: 36422992 Free PMC article. Review.
-
Engineered organoids in oral and maxillofacial regeneration.iScience. 2022 Dec 7;26(1):105757. doi: 10.1016/j.isci.2022.105757. eCollection 2023 Jan 20. iScience. 2022. PMID: 36590157 Free PMC article. Review.
-
Stem cells for organoids.Smart Med. 2022 Dec 27;1(1):e20220007. doi: 10.1002/SMMD.20220007. eCollection 2022 Dec. Smart Med. 2022. PMID: 39188738 Free PMC article. Review.
-
BuZhong YiQi Formula Alleviates Taste Disorders in Rats with Type 2 Diabetes Mellitus by Increasing the Number of Taste Buds and the Expression of Signaling Molecules in Taste Transduction Pathways.Pharmaceuticals (Basel). 2025 Jun 3;18(6):838. doi: 10.3390/ph18060838. Pharmaceuticals (Basel). 2025. PMID: 40573235 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

