A Brief Overview of Global Trends in MSC-Based Cell Therapy
- PMID: 35344199
- PMCID: PMC8958818
- DOI: 10.1007/s12015-022-10369-1
A Brief Overview of Global Trends in MSC-Based Cell Therapy
Abstract
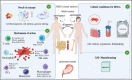
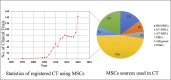
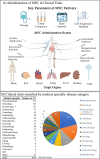
Human mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells or medicinal signaling cells, are important adult stem cells for regenerative medicine, largely due to their regenerative characteristics such as self-renewal, secretion of trophic factors, and the capability of inducing mesenchymal cell lineages. MSCs also possess homing and trophic properties modulating immune system, influencing microenvironment around damaged tissues and enhancing tissue repair, thus offering a broad perspective in cell-based therapies. Therefore, it is not surprising that MSCs have been the broadly used adult stem cells in clinical trials. To gain better insights into the current applications of MSCs in clinical applications, we perform a comprehensive review of reported data of MSCs clinical trials conducted globally. We summarize the biological effects and mechanisms of action of MSCs, elucidating recent clinical trials phases and findings, highlighting therapeutic effects of MSCs in several representative diseases, including neurological, musculoskeletal diseases and most recent Coronavirus infectious disease. Finally, we also highlight the challenges faced by many clinical trials and propose potential solutions to streamline the use of MSCs in routine clinical applications and regenerative medicine.
Keywords: Gene and Stem Cell Therapy; Personalized Medicine; Precision Medicine; Regenerative Medicine; Stem Cell Therapy; iPSC.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

