Ornithine decarboxylase deficiency critically impairs nitrogen metabolism and survival in Aedes aegypti mosquitoes
- PMID: 35344219
- PMCID: PMC8969881
- DOI: 10.1096/fj.202200008R
Ornithine decarboxylase deficiency critically impairs nitrogen metabolism and survival in Aedes aegypti mosquitoes
Abstract
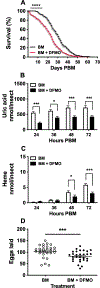
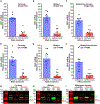
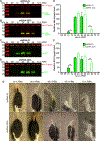
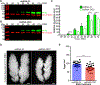
Ornithine decarboxylase (ODC; EC 4.1.1.17) catalyzes the conversion of ornithine to putrescine, the rate-limiting first step for de novo polyamine biosynthesis. Previously, we reported that genetic knockdown of xanthine dehydrogenase 1 (XDH1)-a gene encoding the enzyme involved in the last two steps of uric acid synthesis-causes an increase in ODC transcript levels in fat body of blood-fed Aedes aegypti mosquitoes, suggesting a crosstalk at molecular level between XDH1 and ODC during nitrogen disposal. To further investigate the role of ODC in nitrogen metabolism, we conducted several biochemical and genetic analyses in sugar- and blood-fed A. aegypti females. Distinct ODC gene and protein expression patterns were observed in mosquito tissues dissected during the first gonotrophic cycle. Both pharmacological and RNA interference-mediated knockdown of ODC negatively impacted mosquito survival, disrupted nitrogen waste disposal, delayed oviposition onset, and decreased fecundity in vitellogenic blood-fed females. A lag in the expression of two major digestive serine proteases, a reduction of blood meal digestion in the midgut, and a decrease in vitellogenin yolk protein uptake in ovarian follicles were observed by western blots in ODC-deficient females. Moreover, genetic silencing of ODC showed a broad transcriptional modulation of genes encoding proteins involved in multiple metabolic pathways in mosquito fat body, midgut, and Malpighian tubules prior to and after blood feeding. All together, these data demonstrate that ODC plays an essential role in mosquito metabolism, and that ODC crosstalks with multiple genes and proteins to prevent deadly nitrogen perturbations in A. aegypti females.
Keywords: ammonia metabolism; glucose metabolism; oxidative stress; polyamines; survival.
© 2022 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Figures



Similar articles
-
Xanthine dehydrogenase-1 silencing in Aedes aegypti mosquitoes promotes a blood feeding-induced adulticidal activity.FASEB J. 2017 Jun;31(6):2276-2286. doi: 10.1096/fj.201601185R. Epub 2017 Feb 8. FASEB J. 2017. PMID: 28179423 Free PMC article.
-
Effective disposal of nitrogen waste in blood-fed Aedes aegypti mosquitoes requires alanine aminotransferase.FASEB J. 2016 Jan;30(1):111-20. doi: 10.1096/fj.15-277087. Epub 2015 Aug 26. FASEB J. 2016. PMID: 26310269 Free PMC article.
-
Polyamine metabolism and growth of neurospora strains lacking Cis-acting control sites in the ornithine decarboxylase gene.Arch Biochem Biophys. 1994 Nov 15;315(1):153-60. doi: 10.1006/abbi.1994.1484. Arch Biochem Biophys. 1994. PMID: 7979392
-
Translational regulation of ornithine decarboxylase and other enzymes of the polyamine pathway.Int J Biochem Cell Biol. 1999 Jan;31(1):107-22. doi: 10.1016/s1357-2725(98)00135-6. Int J Biochem Cell Biol. 1999. PMID: 10216947 Review.
-
Degradation of ornithine decarboxylase by the 26S proteasome.Biochem Biophys Res Commun. 2000 Jan 7;267(1):1-6. doi: 10.1006/bbrc.1999.1706. Biochem Biophys Res Commun. 2000. PMID: 10623564 Review.
Cited by
-
Pyruvate kinase is post-translationally regulated by sirtuin 2 in Aedes aegypti mosquitoes.Insect Biochem Mol Biol. 2023 Nov;162:104015. doi: 10.1016/j.ibmb.2023.104015. Epub 2023 Oct 4. Insect Biochem Mol Biol. 2023. PMID: 37797713 Free PMC article.
-
Biochemical and physiological characterization of Aedes aegypti midgut chymotrypsin.Sci Rep. 2025 Mar 20;15(1):9685. doi: 10.1038/s41598-025-93413-7. Sci Rep. 2025. PMID: 40113878 Free PMC article.
-
Comprehensive proteolytic profiling of Aedes aegypti mosquito midgut extracts: Unraveling the blood meal protein digestion system.PLoS Negl Trop Dis. 2025 Feb 6;19(2):e0012555. doi: 10.1371/journal.pntd.0012555. eCollection 2025 Feb. PLoS Negl Trop Dis. 2025. PMID: 39913535 Free PMC article.
References
-
- Nishimura K, Okudaira H, Ochiai E, et al. Identification of proteins whose synthesis is preferentially enhanced by polyamines at the level of translation in mammalian cells. Int J Biochem Cell Biol 2009;41:2251–2261. - PubMed
-
- Rider JE, Hacker A, Mackintosh CA, et al. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007;33:231–240. - PubMed
-
- Bae DH, Lane DJR, Jansson PJ, Richardson DR. The old and new biochemistry of polyamines. Biochim Biophys Acta Gen Subj 2018;1862:2053–2068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

