Cyclic GMP-AMP synthase contributes to epithelial homeostasis in intestinal inflammation via Beclin-1-mediated autophagy
- PMID: 35344224
- PMCID: PMC9040047
- DOI: 10.1096/fj.202200138R
Cyclic GMP-AMP synthase contributes to epithelial homeostasis in intestinal inflammation via Beclin-1-mediated autophagy
Abstract
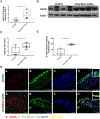
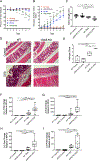
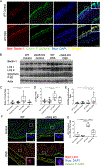
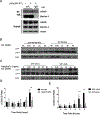
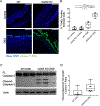
Inflammatory bowel disease (IBD) represents a set of idiopathic and chronic inflammatory diseases of the gastrointestinal tract. Central to the pathogenesis of IBD is a dysregulation of normal intestinal epithelial homeostasis. cGAS is a DNA-sensing receptor demonstrated to promote autophagy, a mechanism that removes dysfunctional cellular components. Beclin-1 is a crucial protein involved in the initiation of autophagy. We hypothesized that cGAS plays a key role in intestinal homeostasis by upregulating Beclin-1-mediated autophagy. We evaluated intestinal cGAS levels in humans with IBD and in murine colonic tissue after performing a 2% dextran sulfate sodium (DSS) colitis model. Autophagy and cell death mechanisms were studied in cGAS KO and WT mice via qPCR, WB analysis, H&E, IF, and TUNEL staining. Autophagy was measured in stimulated intestinal epithelial cells (IECs) via WB analysis. Our data demonstrates cGAS to be upregulated during human and murine colitis. Furthermore, cGAS deficiency leads to worsened colitis and decreased levels of autophagy proteins including Beclin-1 and LC3-II. Co-IP demonstrates a direct binding between cGAS and Beclin-1 in IECs. Transfection of cGAS in stimulated HCT-116 cells leads to increased autophagy. IECs isolated from cGAS KO have diminished autophagic flux. cGAS KO mice subjected to DSS have increased cell death and cleaved caspase-3. Lastly, treatment of cGAS KO mice with rapamycin decreased the severity of colitis. Our data suggest that cGAS maintains intestinal epithelial homeostasis during human IBD and murine colitis by upregulating Beclin-1-mediated autophagy and preventing IEC death. Rescue of autophagy can attenuate the severity of colitis associated with cGAS deficiency.
Keywords: DSS; cGAS; colitis; intestinal epithelium.
© 2022 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Figures




Similar articles
-
New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD.Autophagy. 2020 Jan;16(1):38-51. doi: 10.1080/15548627.2019.1635384. Epub 2019 Jul 9. Autophagy. 2020. PMID: 31286804 Free PMC article. Review.
-
SCFAs induce autophagy in intestinal epithelial cells and relieve colitis by stabilizing HIF-1α.J Mol Med (Berl). 2020 Aug;98(8):1189-1202. doi: 10.1007/s00109-020-01947-2. Epub 2020 Jul 22. J Mol Med (Berl). 2020. PMID: 32696223
-
Epithelial CRL4DCAF2 Is Critical for Maintaining Intestinal Homeostasis Against DSS-Induced Colitis by Regulating the Proliferation and Repair of Intestinal Epithelial Cells.Dig Dis Sci. 2024 Jan;69(1):66-80. doi: 10.1007/s10620-023-08147-1. Epub 2023 Nov 15. Dig Dis Sci. 2024. PMID: 37968554
-
ESRRA (estrogen related receptor alpha) is a critical regulator of intestinal homeostasis through activation of autophagic flux via gut microbiota.Autophagy. 2021 Oct;17(10):2856-2875. doi: 10.1080/15548627.2020.1847460. Epub 2020 Dec 15. Autophagy. 2021. PMID: 33172329 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
T Cell-Derived Apoptotic Extracellular Vesicles Hydrolyze cGAMP to Alleviate Radiation Enteritis via Surface Enzyme ENPP1.Adv Sci (Weinh). 2024 Aug;11(31):e2401634. doi: 10.1002/advs.202401634. Epub 2024 Jun 18. Adv Sci (Weinh). 2024. PMID: 38888507 Free PMC article.
-
Autophagy: a double-edged sword in ischemia-reperfusion injury.Cell Mol Biol Lett. 2025 Apr 7;30(1):42. doi: 10.1186/s11658-025-00713-x. Cell Mol Biol Lett. 2025. PMID: 40197222 Free PMC article. Review.
-
Intestinal epithelial CGAS dampens inflammation by upregulating autophagy.Autophagy Rep. 2022 Jul 19;1(1):306-308. doi: 10.1080/27694127.2022.2098305. eCollection 2022. Autophagy Rep. 2022. PMID: 40396049 Free PMC article.
-
Exploring the anticancer potential of nitrated N-substituted-4-hydroxy-2-quinolone-3-carboxamides: synthesis, biological assessment, and computational analysis.BMC Chem. 2025 Aug 22;19(1):247. doi: 10.1186/s13065-025-01616-w. BMC Chem. 2025. PMID: 40846973 Free PMC article.
-
cGAS-STING pathway as a promising target for digestive diseases: insights from natural plant products.Front Nutr. 2025 Jun 20;12:1594120. doi: 10.3389/fnut.2025.1594120. eCollection 2025. Front Nutr. 2025. PMID: 40621423 Free PMC article. Review.
References
-
- De Souza HSP, Fiocchi C. Immunopathogenesis of IBD: Current State of the Art. Nat Rev Gastroenterol Hepatol. 2016. January;13(1):13–27. - PubMed
-
- Kaplan GG. The Global Gurden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015. December;12(12):720–7. - PubMed
-
- Rampton DS, Shanahan F. Fast Facts: Inflammatory Bowel Disease. Kargers Publication; Health Press, 2016.
-
- Cavlar T, Ablasser A, Hornung V. Induction of Type I IFNs by Intracellular DNA-Sensing Pathways. Immunol Cell Biol. 2012. May;90(5):474–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

