Blockage of MLKL prevents myelin damage in experimental diabetic neuropathy
- PMID: 35344427
- PMCID: PMC9168919
- DOI: 10.1073/pnas.2121552119
Blockage of MLKL prevents myelin damage in experimental diabetic neuropathy
Abstract
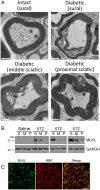
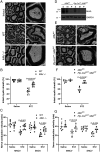
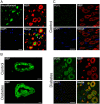
SignificanceDiabetic neuropathy is a commonly occurring complication of diabetes that affects hundreds of millions of patients worldwide. Patients suffering from diabetic neuropathy experience abnormal sensations and have damage in their peripheral nerve axons as well as myelin, a tightly packed Schwann cell sheath that wraps around axons to provide insulation and increases electrical conductivity along the nerve fibers. The molecular events underlying myelin damage in diabetic neuropathy are largely unknown, and there is no efficacious treatment for the disease. The current study, using a diabetic mouse model and human patient nerve samples, uncovered a molecular mechanism underlying myelin sheath damage in diabetic neuropathy and provides a potential treatment strategy for the disease.
Keywords: MLKL; diabetic neuropathy; myelin.
Conflict of interest statement
The authors declare no competing interest.
Figures


Comment in
-
Schwann cell necroptosis in diabetic neuropathy.Proc Natl Acad Sci U S A. 2022 Apr 26;119(17):e2204049119. doi: 10.1073/pnas.2204049119. Epub 2022 Apr 21. Proc Natl Acad Sci U S A. 2022. PMID: 35446622 Free PMC article. No abstract available.
Similar articles
-
Taurine protects against myelin damage of sciatic nerve in diabetic peripheral neuropathy rats by controlling apoptosis of schwann cells via NGF/Akt/GSK3β pathway.Exp Cell Res. 2019 Oct 15;383(2):111557. doi: 10.1016/j.yexcr.2019.111557. Epub 2019 Aug 12. Exp Cell Res. 2019. PMID: 31415759
-
Mixed Lineage Kinase Domain-like Protein MLKL Breaks Down Myelin following Nerve Injury.Mol Cell. 2018 Nov 1;72(3):457-468.e5. doi: 10.1016/j.molcel.2018.09.011. Epub 2018 Oct 18. Mol Cell. 2018. PMID: 30344099
-
Schwann Cells as Crucial Players in Diabetic Neuropathy.Adv Exp Med Biol. 2019;1190:345-356. doi: 10.1007/978-981-32-9636-7_22. Adv Exp Med Biol. 2019. PMID: 31760655 Review.
-
Role of the Schwann cell in diabetic neuropathy.Int Rev Neurobiol. 2002;50:293-321. doi: 10.1016/s0074-7742(02)50081-7. Int Rev Neurobiol. 2002. PMID: 12198814 Review.
-
Blocking mitochondrial calcium release in Schwann cells prevents demyelinating neuropathies.J Clin Invest. 2016 Mar 1;126(3):1023-38. doi: 10.1172/JCI84505. Epub 2016 Feb 15. J Clin Invest. 2016. Retraction in: J Clin Invest. 2017 Mar 1;127(3):1115. doi: 10.1172/JCI92100. PMID: 26878172 Free PMC article. Retracted.
Cited by
-
PEX11B palmitoylation couples peroxisomal dysfunction with Schwann cells fail in diabetic neuropathy.J Biomed Sci. 2025 Feb 12;32(1):20. doi: 10.1186/s12929-024-01115-5. J Biomed Sci. 2025. PMID: 39934809 Free PMC article.
-
Unveiling the Role of Schwann Cell Plasticity in the Pathogenesis of Diabetic Peripheral Neuropathy.Int J Mol Sci. 2024 Oct 8;25(19):10785. doi: 10.3390/ijms251910785. Int J Mol Sci. 2024. PMID: 39409114 Free PMC article. Review.
-
Dysregulated mast cell activation induced by diabetic milieu exacerbates the progression of diabetic peripheral neuropathy in mice.Nat Commun. 2025 May 5;16(1):4170. doi: 10.1038/s41467-025-59562-z. Nat Commun. 2025. PMID: 40325050 Free PMC article.
-
MLKL‒OPTN axis regulates herpesvirus-induced neurological sequelae.Clin Transl Med. 2025 Jun;15(6):e70353. doi: 10.1002/ctm2.70353. Clin Transl Med. 2025. PMID: 40490945 Free PMC article.
-
Mixed lineage kinase domain-like protein in liver diseases: Cell-type-specific functions and dual roles.World J Gastroenterol. 2025 Apr 14;31(14):104523. doi: 10.3748/wjg.v31.i14.104523. World J Gastroenterol. 2025. PMID: 40248377 Free PMC article.
References
-
- International Diabetes Federation, IDF Diabetes Atlas (IDF, ed. 10, 2021). https://diabetesatlas.org/atlas/tenth-edition/. Accessed 9 January 2022. - PubMed
-
- Feldman E. L., et al. , Diabetic neuropathy. Nat. Rev. Dis. Primers 5, 41 (2019). - PubMed
-
- Fernyhough P., Mitochondrial dysfunction in diabetic neuropathy: A series of unfortunate metabolic events. Curr. Diab. Rep. 15, 89 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

