Etiology of pediatric lower respiratory tract infections in South Korea
- PMID: 35344458
- PMCID: PMC9196667
- DOI: 10.1080/21645515.2022.2048579
Etiology of pediatric lower respiratory tract infections in South Korea
Abstract
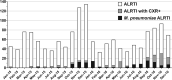
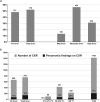
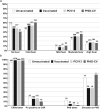
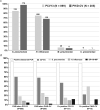
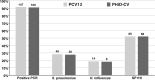
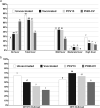
Lower respiratory tract infections (LRTIs) are an important cause of death and bacterial pneumonia is one of the most common causes of mortality in South Korea, but there is little data evaluating the epidemiology of pediatric LRTI in primary care clinics. We evaluated 1,497 pediatric LRTI cases in a primary care clinic over a two-year period from 2015 to 16 for clinical and radiological signs combined with PCR for pathogen detection. In addition, a 1,837 vaccine cohort in the clinic from 2014 to 16 was analyzed separately. Fifty-two percent of cases presented with fever and 15% of 1,423 X-rayed cases had positive pneumonia findings with the grade of fever correlating positively with the proportion of cases with positive chest findings. Bacterial identification was possible for 1,376 cases with Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae most common. A higher proportion of 13-valent pneumococcal conjugate vaccinated cases had positive pneumonia findings than 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) vaccinated cases, although similar proportions for each PCV had confirmed bacterial infections. PHiD-CV vaccinated cases with positive pneumonia findings had proportionally more single S. pneumoniae infections but less co-infections and less cases with H. influenzae infection. The proportions of confirmed bacterial infections in LRTI cases observed in this pediatric primary care setting in South Korea is very high, with co-infections most common. S. pneumoniae and H. influenzae are the most common as expected but this data also highlights M. pneumoniae as an additional important cause of LRTI in primary pediatric care in Korea.
Keywords: LRTI; Pneumococcal conjugate vaccines; Pneumonia; etiology; primary care.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Cost-effectiveness analysis of infant pneumococcal vaccination with PHiD-CV in Korea.Hum Vaccin Immunother. 2018 Jan 2;14(1):85-94. doi: 10.1080/21645515.2017.1362513. Epub 2017 Nov 8. Hum Vaccin Immunother. 2018. PMID: 29115905 Free PMC article.
-
Reduced nontypeable Haemophilus influenzae lower airway infection in children with chronic endobronchial suppuration vaccinated with the 10-valent pneumococcal H. influenzae protein D conjugate vaccine.Vaccine. 2018 Mar 20;36(13):1736-1742. doi: 10.1016/j.vaccine.2018.02.054. Epub 2018 Feb 23. Vaccine. 2018. PMID: 29478754
-
Immunogenicity and safety of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine (PHiD-CV) co-administered with routine childhood vaccines in Taiwan.J Formos Med Assoc. 2012 Sep;111(9):495-503. doi: 10.1016/j.jfma.2011.07.014. Epub 2012 Mar 18. J Formos Med Assoc. 2012. PMID: 23021506 Clinical Trial.
-
10-Valent pneumococcal non-typeable haemophilus influenzae protein D-conjugate vaccine: a review in infants and children.Paediatr Drugs. 2014 Oct;16(5):425-44. doi: 10.1007/s40272-014-0089-x. Paediatr Drugs. 2014. PMID: 25192686 Review.
-
A systematic review of invasive pneumococcal disease vaccine failures and breakthrough with higher-valency pneumococcal conjugate vaccines in children.Expert Rev Vaccines. 2022 Feb;21(2):201-214. doi: 10.1080/14760584.2022.2012455. Epub 2022 Feb 3. Expert Rev Vaccines. 2022. PMID: 34882050
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
