Placental nutrient transporters adapt during persistent maternal hypoglycaemia in rats
- PMID: 35344549
- PMCID: PMC8959168
- DOI: 10.1371/journal.pone.0265988
Placental nutrient transporters adapt during persistent maternal hypoglycaemia in rats
Abstract
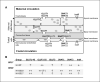
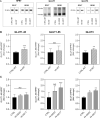
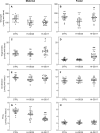
Maternal malnutrition is associated with decreased nutrient transfer to the foetus, which may lead to foetal growth restriction, predisposing children to a variety of diseases. However, regulation of placental nutrient transfer during decreased nutrient availability is not fully understood. In the present study, the aim was to investigate changes in levels of placental nutrient transporters accompanying maternal hypoglycaemia following different durations and stages of gestation in rats. Maternal hypoglycaemia was induced by insulin-infusion throughout gestation until gestation day (GD)20 or until end of organogenesis (GD17), with sacrifice on GD17 or GD20. Protein levels of placental glucose transporters GLUT1 (45/55 kDa isotypes) and GLUT3, amino acid transporters SNAT1 and SNAT2, and insulin receptor (InsR) were assessed. On GD17, GLUT1-45, GLUT3, and SNAT1 levels were increased and InsR levels decreased versus controls. On GD20, following hypoglycaemia throughout gestation, GLUT3 levels were increased, GLUT1-55 showed the same trend. After cessation of hypoglycaemia at end of organogenesis, GLUT1-55, GLUT3, and InsR levels were increased versus controls, whereas SNAT1 levels were decreased. The increases in levels of placental nutrient transporters seen during maternal hypoglycaemia and hyperinsulinemia likely reflect an adaptive response to optimise foetal nutrient supply and development during limited availability of glucose.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport.J Physiol. 2007 Jun 15;581(Pt 3):1323-32. doi: 10.1113/jphysiol.2007.131185. Epub 2007 Apr 12. J Physiol. 2007. PMID: 17430988 Free PMC article.
-
Maternal undernutrition during late gestation-induced intrauterine growth restriction in the rat is associated with impaired placental GLUT3 expression, but does not correlate with endogenous corticosterone levels.J Endocrinol. 2002 Jul;174(1):37-43. doi: 10.1677/joe.0.1740037. J Endocrinol. 2002. PMID: 12098661
-
Placental glucose and amino acid transport in calorie-restricted wild-type and Glut3 null heterozygous mice.Endocrinology. 2012 Aug;153(8):3995-4007. doi: 10.1210/en.2011-1973. Epub 2012 Jun 14. Endocrinology. 2012. PMID: 22700768 Free PMC article.
-
Effect of maternal hypoglycaemia during gestation on materno-foetal nutrient transfer and embryo-foetal development: Evidence from experimental studies focused primarily on the rat.Reprod Toxicol. 2018 Apr;77:1-24. doi: 10.1016/j.reprotox.2018.01.007. Epub 2018 Feb 3. Reprod Toxicol. 2018. PMID: 29408374 Review.
-
Placental transport of nutrients and its implications for fetal growth.J Reprod Fertil Suppl. 1999;54:401-10. J Reprod Fertil Suppl. 1999. PMID: 10692871 Review.
References
-
- Malhotra A, Allison BJ, Castillo-Melendez M, Jenkin G, Polglase GR, Miller SL. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Frontiers in endocrinology. 2019;10:55. Epub 2019/02/23. doi: 10.3389/fendo.2019.00055 ; PubMed Central PMCID: PMC6374308. - DOI - PMC - PubMed
-
- Lee AC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, et al.. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: analysis of CHERG datasets. BMJ (Clinical research ed). 2017;358:j3677. Epub 2017/08/19. doi: 10.1136/bmj.j3677 ; PubMed Central PMCID: PMC5558898 at www.icmje.org/coi_disclosure.pdf and declare: financial support for the submitted work from the Bill and Melinda Gates Foundation by a grant to the US Fund for UNICEF; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. - DOI - PMC - PubMed
-
- Jensen VFH, Molck AM, Lykkesfeldt J, Bogh IB. Effect of maternal hypoglycaemia during gestation on materno-foetal nutrient transfer and embryo-foetal development: Evidence from experimental studies focused primarily on the rat. Reproductive toxicology (Elmsford, NY). 2018;77:1–24. Epub 2018/02/07. doi: 10.1016/j.reprotox.2018.01.007 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

