Fluorescence of KCl Aqueous Solution: A Possible Spectroscopic Signature of Nucleation
- PMID: 35344657
- PMCID: PMC8996234
- DOI: 10.1021/acs.jpcb.2c01496
Fluorescence of KCl Aqueous Solution: A Possible Spectroscopic Signature of Nucleation
Abstract
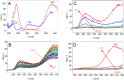
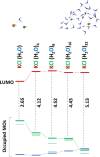
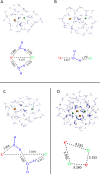
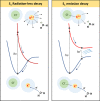
Ion pairing in water solutions alters both the water hydrogen-bond network and ion solvation, modifying the dynamics and properties of electrolyte water solutions. Here, we report an anomalous intrinsic fluorescence of KCl aqueous solution at room temperature and show that its intensity increases with the salt concentration. From the ab initio density functional theory (DFT) and time-dependent DFT modeling, we propose that the fluorescence emission could originate from the stiffening of the hydrogen bond network in the hydration shell of solvated ion-pairs that suppresses the fast nonradiative decay and allows the slower radiative channel to become a possible decay pathway. Because computations suggest that the fluorophores are the local ion-water structures present in the prenucleation phase, this band could be the signature of the incoming salt precipitation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Anomalous Intrinsic Fluorescence of HCl and NaOH Aqueous Solutions.J Phys Chem Lett. 2019 Nov 21;10(22):7230-7236. doi: 10.1021/acs.jpclett.9b02163. Epub 2019 Nov 11. J Phys Chem Lett. 2019. PMID: 31689111
-
Density functional theory based molecular dynamics study of solution composition effects on the solvation shell of metal ions.Phys Chem Chem Phys. 2020 Jul 22;22(28):16301-16313. doi: 10.1039/d0cp01957g. Phys Chem Chem Phys. 2020. PMID: 32647838
-
Hofmeister anionic effects on hydration electric fields around water and peptide.J Chem Phys. 2012 Mar 28;136(12):124501. doi: 10.1063/1.3694036. J Chem Phys. 2012. PMID: 22462868
-
Linking electronic and molecular structure: insight into aqueous chloride solvation.Phys Chem Chem Phys. 2013 Aug 21;15(31):13169-83. doi: 10.1039/c3cp50652e. Phys Chem Chem Phys. 2013. PMID: 23824018
-
Ab Initio Molecular Dynamics Study of Aqueous Solutions of Magnesium and Calcium Nitrates: Hydration Shell Structure, Dynamics and Vibrational Echo Spectroscopy.J Phys Chem B. 2022 Jan 20;126(2):528-544. doi: 10.1021/acs.jpcb.1c08545. Epub 2022 Jan 8. J Phys Chem B. 2022. PMID: 35001626
Cited by
-
Anomalous Water Fluorescence Induced by Solutes.J Phys Chem Lett. 2025 Jul 10;16(27):6935-6945. doi: 10.1021/acs.jpclett.5c01189. Epub 2025 Jun 30. J Phys Chem Lett. 2025. PMID: 40586586 Free PMC article.
-
Bimodal Ionic Conduction through Polymer Films due to Nano Confinement.Angew Chem Int Ed Engl. 2025 Apr 17;64(17):e202423548. doi: 10.1002/anie.202423548. Epub 2025 Mar 6. Angew Chem Int Ed Engl. 2025. PMID: 39994681 Free PMC article. Review.
References
-
- Zimbitas G.; Jawor-Baczynska A.; Vesga M. J.; Javid N.; Moore B. D.; Parkinson J.; Sefcik J. Investigation of Molecular and Mesoscale Clusters in Undersaturated Glycine Aqueous Solutions. Colloids Surf., A 2019, 579, 123633.10.1016/j.colsurfa.2019.123633. - DOI
-
- Georgalis Y.; Kierzek A. M.; Saenger W. Cluster Formation in Aqueous Electrolyte Solutions Observed by Dynamic Light Scattering. J. Phys. Chem. B 2000, 104, 3405–3406. 10.1021/jp000132e. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

