Pathophysiological analysis of uninephrectomized db/db mice as a model of severe diabetic kidney disease
- PMID: 35344670
- PMCID: PMC9150550
- DOI: 10.33549/physiolres.934784
Pathophysiological analysis of uninephrectomized db/db mice as a model of severe diabetic kidney disease
Abstract
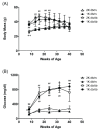
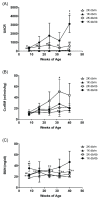
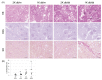
Diabetic nephropathy, included in diabetic kidney disease (DKD), is the primary disease leading to end-stage renal disease (ESRD) or dialysis treatment, accounting for more than 40% of all patients with ESRD or receiving dialysis. Developing new therapeutics to prevent the transition to ESRD or dialysis treatment requires an understanding of the pathophysiology of DKD and an appropriate animal model for drug efficacy studies. In this study, we investigated the pathophysiology of diabetic kidney disease with type 2 diabetes in uninephrectomized db/db mice. In addition, the nephrectomized db /db mice from 10 weeks to 42 weeks were used to assess the efficacy of long-term administration of the angiotensin-II-receptor antagonist losartan. The blood and urinary biochemical parameters, main pharmacological endpoint of the losartan therapy, were periodically measured. And at the end, histopathological analysis was performed. Uninephrectomized db/db mice clearly developed obesity and hyperglycemia from young age. Furthermore, they showed renal pathophysiological changes, such as increased urinary albumin-creatinine ratio (UACR) (the peak value 3104 ± 986 in 40-week-old mice), glomerular hypertrophy and increased fibrotic areas in the tubulointerstitial tubules. The blood pressure in the losartan group was significantly low compared to the normotensive Vehicle group. However, as expected, Losartan suppressed the increase in UACR (829±500) indicating the medication was sufficient, but the histopathological abnormalities including tubular interstitial fibrosis did not improve. These results suggest that the uninephrectomized db/db mice are useful as an animal model of the severe DKD indicated by the comparison of the efficacy of losartan in this model with the efficacy of losartan in clinical practice.
Conflict of interest statement
M. Maekawa, T. Maekawa, Sasase, Takagi, Takeuchi, Kitamoto, Nakagawa, Toyoda and Konishi are employees of Japan Tobacco Inc.
Figures
References
-
- Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2018;71:A7. doi: 10.1053/j.ajkd.2018.01.002. - DOI - PMC - PubMed
-
- Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73:A7–A8. doi: 10.1053/j.ajkd.2019.01.001. - DOI - PMC - PubMed
-
- Japanese Society of Nephrology. Clinical Practice Guidebook for Diagnosis and Treatment of Chronic Kidney Disease 2012. Tokyo Igakusha; Tokyo: 2012.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
