The association between inadequate sleep and accelerated brain ageing
- PMID: 35344818
- PMCID: PMC9084918
- DOI: 10.1016/j.neurobiolaging.2022.02.005
The association between inadequate sleep and accelerated brain ageing
Abstract
Numerous studies indicate large heterogeneity in brain ageing, which can be attributed to modifiable lifestyle factors, including sleep. Inadequate sleep has been previously linked to gray (GM) and white (WM) matter changes. However, the reported findings are highly inconsistent. By contrast to previous research independently characterizing patterns of either GM or WM changes, we used here linked independent component analysis (FLICA) to examine covariation in GM, and WM in a group of older adults (n = 50). Next, we employed a novel technique to estimate the brain age delta (difference between chronological and brain age assessed using neuroimaging data) and study its associations with sleep quality and sleep fragmentation, hypothesizing that inadequate sleep accelerates brain ageing. FLICA revealed a number of multimodal components, associated with age, sleep quality, and sleep fragmentation. Subsequently, we show significant associations between brain age delta and inadequate sleep, suggesting 2 years deviation above the chronological age. Our findings indicate sensitivity of multimodal approaches and brain age delta in detecting link between inadequate sleep and accelerated brain ageing.
Keywords: Ageing; Brain age; Gray matter; Magnetic resonance imaging; Sleep; White matter.
Copyright © 2022. Published by Elsevier Inc.
Figures

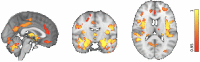
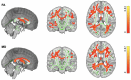
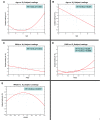

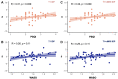
Similar articles
-
The association of regional white matter lesions with cognition in a community-based cohort of older individuals.Neuroimage Clin. 2018 Mar 29;19:14-21. doi: 10.1016/j.nicl.2018.03.035. eCollection 2018. Neuroimage Clin. 2018. PMID: 30034997 Free PMC article.
-
Association of Blood Pressure With Brain Ages: A Cohort Study of Gray and White Matter Aging Discrepancy in Mid-to-Older Adults From UK Biobank.Hypertension. 2024 Apr;81(4):906-916. doi: 10.1161/HYPERTENSIONAHA.123.22176. Epub 2024 Mar 11. Hypertension. 2024. PMID: 38465593
-
Regional glucose metabolic decreases with ageing are associated with microstructural white matter changes: a simultaneous PET/MR study.Eur J Nucl Med Mol Imaging. 2022 Jan;49(2):664-680. doi: 10.1007/s00259-021-05518-6. Epub 2021 Aug 16. Eur J Nucl Med Mol Imaging. 2022. PMID: 34398271
-
Obstructive Sleep Apnea and the Brain: a Focus on Gray and White Matter Structure.Curr Neurol Neurosci Rep. 2021 Feb 14;21(3):11. doi: 10.1007/s11910-021-01094-2. Curr Neurol Neurosci Rep. 2021. PMID: 33586028 Review.
-
Relationship Between White Matter Lesions and Gray Matter Atrophy in Multiple Sclerosis: A Systematic Review.Neurology. 2022 Apr 12;98(15):e1562-e1573. doi: 10.1212/WNL.0000000000200006. Epub 2022 Feb 16. Neurology. 2022. PMID: 35173016 Free PMC article.
Cited by
-
Meditation Experience is Associated with Increased Structural Integrity of the Pineal Gland and greater total Grey Matter maintenance.medRxiv [Preprint]. 2024 Mar 5:2024.03.04.24303649. doi: 10.1101/2024.03.04.24303649. medRxiv. 2024. PMID: 38496551 Free PMC article. Preprint.
-
A systematic review of multimodal brain age studies: Uncovering a divergence between model accuracy and utility.Patterns (N Y). 2023 Apr 14;4(4):100712. doi: 10.1016/j.patter.2023.100712. eCollection 2023 Apr 14. Patterns (N Y). 2023. PMID: 37123443 Free PMC article. Review.
-
Comprehensive assessment of sleep duration, insomnia, and brain structure within the UK Biobank cohort.Sleep. 2024 Feb 8;47(2):zsad274. doi: 10.1093/sleep/zsad274. Sleep. 2024. PMID: 37889226 Free PMC article.
-
Associations between polygenic risk scores and accelerated brain ageing in smokers.Psychol Med. 2023 Dec;53(16):7785-7794. doi: 10.1017/S0033291723001812. Epub 2023 Aug 9. Psychol Med. 2023. PMID: 37555321 Free PMC article.
-
Sex-difference in the association between social drinking, structural brain aging and cognitive function in older individuals free of cognitive impairment.Front Psychiatry. 2024 Apr 8;15:1235171. doi: 10.3389/fpsyt.2024.1235171. eCollection 2024. Front Psychiatry. 2024. PMID: 38651011 Free PMC article.
References
-
- Alfaro-Almagro F., Jenkinson M., Bangerter N.K., Andersson J.L.R., Griffanti L., Douaud G., Sotiropoulos S.N., Jbabdi S., Hernandez-Fernandez M., Vallee E., Vidaurre D., Webster M., McCarthy P., Rorden C., Daducci A., Alexander D.C., Zhang H., Dragonu I., Matthews P.M., Miller K.L., Smith S.M. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. - DOI - PMC - PubMed
-
- Andersson J.L.R., Jenkinson M., Smith S. High resolution nonlinear registration with simultaneous modelling of intensities. bioRxiv. 2019;646802 doi: 10.1101/646802. - DOI
-
- André C., Tomadesso C., de Flores R., Branger P., Rehel S., Mézenge F., Landeau B., Sayette V.d.l., Eustache F., Chételat G., Rauchs G. Brain and cognitive correlates of sleep fragmentation in elderly subjects with and without cognitive deficits. Alzheimers Dement. 2019;11:142–150. doi: 10.1016/j.dadm.2018.12.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

