Static Magnetic Field Promotes Proliferation, Migration, Differentiation, and AKT Activation of Periodontal Ligament Stem Cells
- PMID: 35344952
- PMCID: PMC10534995
- DOI: 10.1159/000524291
Static Magnetic Field Promotes Proliferation, Migration, Differentiation, and AKT Activation of Periodontal Ligament Stem Cells
Abstract
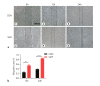
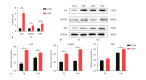
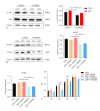
Periodontal ligament stem cells (PDLSCs) possess self-renewal and multilineage differentiation potential and exhibit great potential for the treatment of bone tissue defects caused by inflammation. Previous studies have indicated that static magnetic field (SMF) can enhance the proliferation and differentiation of mesenchymal stem cells (MSCs). SMF has been widely used to repair bone defects and for orthodontic and implantation treatment. In this study, we revealed that a 320 mT SMF upregulates the protein expression levels of cytokines such as MCM7 and PCNA in proliferating PDLSCs. Cell counting kit-8 results revealed that the SMF group had higher optical density values than the control group. The ratio of cells in the S phase to those in the G2/M phase was significantly increased after exposure to a 320 mT SMF. In scratch assays, the SMF-treated PDLSCs exhibited a higher migration rate than the sham-exposed group after 24 h of culture, indicating that the SMF promoted the migratory ability of PDLSCs. The activity level of the early differentiation marker alkaline phosphatase and the late marker matrix mineralization, as well as osteoblast-specific gene and protein expression, were enhanced in PDLSCs exposed to the SMF. Furthermore, AKT signaling pathway was activated by SMF. Our data demonstrated that the potential mechanism of action of SMF may enhance PDLSCs proliferation and osteogenic differentiation by activating the phosphorylated AKT pathway. The elucidation of this molecular mechanism may lead to a better understanding of bone repair responses and aid in improved stem cell-mediated regeneration.
Keywords: Migration; Osteogenic differentiation; Proliferation; Protein kinase B; Static magnetic fields.
© 2022 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Oxytocin facilitates the proliferation, migration and osteogenic differentiation of human periodontal stem cells in vitro.Arch Oral Biol. 2019 Mar;99:126-133. doi: 10.1016/j.archoralbio.2019.01.007. Epub 2019 Jan 19. Arch Oral Biol. 2019. PMID: 30682715
-
Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions.Cell Prolif. 2017 Dec;50(6):e12369. doi: 10.1111/cpr.12369. Epub 2017 Aug 23. Cell Prolif. 2017. PMID: 28833827 Free PMC article.
-
Enamel matrix derivative enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells on the titanium implant surface.Organogenesis. 2017 Jul 3;13(3):103-113. doi: 10.1080/15476278.2017.1331196. Epub 2017 Jun 9. Organogenesis. 2017. PMID: 28598248 Free PMC article.
-
PSAT1 positively regulates the osteogenic lineage differentiation of periodontal ligament stem cells through the ATF4/PSAT1/Akt/GSK3β/β-catenin axis.J Transl Med. 2023 Feb 2;21(1):70. doi: 10.1186/s12967-022-03775-z. J Transl Med. 2023. PMID: 36732787 Free PMC article.
-
Potassium dihydrogen phosphate promotes the proliferation and differentiation of human periodontal ligament stem cells via nuclear factor kappa B pathway.Exp Cell Res. 2019 Nov 1;384(1):111593. doi: 10.1016/j.yexcr.2019.111593. Epub 2019 Sep 2. Exp Cell Res. 2019. PMID: 31487508
Cited by
-
miR-508-5p suppresses osteogenic differentiation of human periodontal ligament stem cells via targeting sex-determining region Y-related HMG-box 11.J Dent Sci. 2025 Jan;20(1):201-211. doi: 10.1016/j.jds.2024.08.012. Epub 2024 Aug 28. J Dent Sci. 2025. PMID: 39873049 Free PMC article.
-
Electrokinetic properties of healthy and β-thalassemia erythrocyte membranes under in vitro exposure to static magnetic field.Front Chem. 2023 Oct 19;11:1197210. doi: 10.3389/fchem.2023.1197210. eCollection 2023. Front Chem. 2023. PMID: 37927566 Free PMC article.
-
Moderate static magnetic field promotes fracture healing and regulates iron metabolism in mice.Biomed Eng Online. 2023 Nov 15;22(1):107. doi: 10.1186/s12938-023-01170-3. Biomed Eng Online. 2023. PMID: 37968671 Free PMC article.
-
Static magnetic field-induced IL-6 secretion in periodontal ligament stem cells accelerates orthodontic tooth movement.Sci Rep. 2024 Apr 29;14(1):9851. doi: 10.1038/s41598-024-60621-6. Sci Rep. 2024. PMID: 38684732 Free PMC article.
-
Moderate static magnetic field regulates iron metabolism and salvage bone loss caused by iron accumulation.J Orthop Translat. 2025 Jan 10;50:144-157. doi: 10.1016/j.jot.2024.10.012. eCollection 2025 Jan. J Orthop Translat. 2025. PMID: 40171108 Free PMC article.
References
-
- Brazil DP, Hemmings BA. Ten years of protein kinase B signaling: A hard Akt to follow. Trends Biochem Sci. 2001 Nov;26((11)):657–664. - PubMed
-
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signaling: AKTion on multiple fronts. Trends Biochem Sci. 2004 May;29((5)):233–242. - PubMed
-
- Chang CY, Lew WZ, Feng SW, Wu CL, Wang HH, Hsieh SC, et al. Static magnetic field-enhanced osteogenic differentiation of human umbilical cord-derived mesenchymal stem cells via matrix vesicle secretion. Int J Radiat Biol. 2020 Sep;96((9)):1207–1217. - PubMed
-
- Denaro V, Papapietro N, Sgambato A, Barnaba SA, Ruzzini L, Paola BD, et al. Periprosthetic electrochemical corrosion of titanium and titanium-based alloys as a cause of spinal fusion failure. Spine. 2008 Jan;33((1)):8–13. - PubMed
-
- Farzaneh S, Hosseinzadeh S, Samanipour R, Hatamie S, Ranjbari J, Khojasteh A. Fabrication and characterization of cobalt ferrite magnetic hydrogel combined with static magnetic field as a potential bio-composite for bone tissue engineering. J Drug Deliv Sci Technol. 2021 May;64:102525.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

