Effects of Pulsatility on Arterial Endothelial and Smooth Muscle Cells
- PMID: 35344966
- PMCID: PMC10782761
- DOI: 10.1159/000524317
Effects of Pulsatility on Arterial Endothelial and Smooth Muscle Cells
Abstract
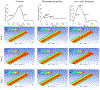
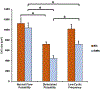
Continuous flow ventricular assist device (CFVAD) support in advanced heart failure patients causes diminished pulsatility, which has been associated with adverse events including gastrointestinal bleeding, end organ failure, and arteriovenous malformation. Recently, pulsatility augmentation by pump speed modulation has been proposed as a means to minimize adverse events. Pulsatility primarily affects endothelial and smooth muscle cells in the vasculature. To study the effects of pulsatility and pulse modulation using CFVADs, we have developed a microfluidic co-culture model with human aortic endothelial (ECs) and smooth muscle cells (SMCs) that can replicate physiologic pressures, flows, shear stresses, and cyclical stretch. The effects of pulsatility and pulse frequency on ECs and SMCs were evaluated during (1) normal pulsatile flow (120/80 mmHg, 60 bpm), (2) diminished pulsatility (98/92 mmHg, 60 bpm), and (3) low cyclical frequency (115/80 mmHg, 30 bpm). Shear stresses were estimated using computational fluid dynamics (CFD) simulations. While average shear stresses (4.2 dynes/cm2) and flows (10.1 mL/min) were similar, the peak shear stresses for normal pulsatile flow (16.9 dynes/cm2) and low cyclic frequency (19.5 dynes/cm2) were higher compared to diminished pulsatility (6.45 dynes/cm2). ECs and SMCs demonstrated significantly lower cell size with diminished pulsatility compared to normal pulsatile flow. Low cyclical frequency resulted in normalization of EC cell size but not SMCs. SMCs size was higher with low frequency condition compared to diminished pulsatility but did not normalize to normal pulsatility condition. These results may suggest that pressure amplitude augmentation may have a greater effect in normalizing ECs, while both pressure amplitude and frequency may be required to normalize SMCs morphology. The co-culture model may be an ideal platform to study flow modulation strategies.
Keywords: Diminished pulsatility; Pulse flow modulation; Vascular model; Ventricular assist device.
© 2022 S. Karger AG, Basel.
Conflict of interest statement
Conflict of Interest Statement
G.A. Giridharan is a consultant for NuPulseCV.
Figures


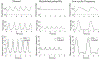

References
-
- Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke. 2005. Feb;36(2):337–41. - PubMed
-
- Bartoli CR, Spence PA, Siess T, Raess DH, Koenig SC, Dowling RD. Nonphysiologic blood flow triggers endothelial and arterial remodeling in vivo: Implications for novel left ventricular assist devices with a peripheral anastomosis. The Journal of Thoracic and Cardiovascular Surgery. 2014;148(1):311–21. - PMC - PubMed
-
- Bartoli CR, Zhang DM, Hennessy-Strahs S, Kang J, Restle DJ, Bermudez C, et al. Clinical and In Vitro Evidence That Left Ventricular Assist Device–Induced von Willebrand Factor Degradation Alters Angiogenesis. Circulation: Heart Failure. 2018;11(9):e004638. - PubMed
-
- Bearnson GB, Olsen DB, Khanwilkar PS, Long JW, Allaire PE, Maslen EH. Pulsatile operation of a centrifugal ventricular assist device with magnetic bearings. ASAIO J. 1996. Sep-Oct;42(5):M620–4. - PubMed
-
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992;86(3):723–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

